| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:34:15 UTC |
|---|
| Update Date | 2016-11-09 01:22:30 UTC |
|---|
| Accession Number | CHEM041559 |
|---|
| Identification |
|---|
| Common Name | 5-(3',4',5'-Trihydroxyphenyl)-gamma-valerolactone |
|---|
| Class | Small Molecule |
|---|
| Description | A butan-4-olide that is gamma-valerolactone in which one of the methyl hydrogens has been replaced by a 3,4,5-trihydroxyphenyl group. |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
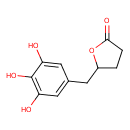
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5-(3',4',5'-Trihydroxyphenyl)-g-valerolactone | Generator | | 5-(3',4',5'-Trihydroxyphenyl)-γ-valerolactone | Generator |
|
|---|
| Chemical Formula | C11H12O5 |
|---|
| Average Molecular Mass | 224.210 g/mol |
|---|
| Monoisotopic Mass | 224.068 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 5-[(3,4,5-trihydroxyphenyl)methyl]oxolan-2-one |
|---|
| Traditional Name | 5-[(3,4,5-trihydroxyphenyl)methyl]oxolan-2-one |
|---|
| SMILES | OC1=CC(CC2CCC(=O)O2)=CC(O)=C1O |
|---|
| InChI Identifier | InChI=1S/C11H12O5/c12-8-4-6(5-9(13)11(8)15)3-7-1-2-10(14)16-7/h4-5,7,12-13,15H,1-3H2 |
|---|
| InChI Key | CZVAQLJEUUFQCH-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyrogallols and derivatives. Pyrogallols and derivatives are compounds containing a 1,2,3-trihydroxybenzene moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenols |
|---|
| Sub Class | Benzenetriols and derivatives |
|---|
| Direct Parent | Pyrogallols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrogallol derivative
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- Gamma butyrolactone
- Tetrahydrofuran
- Carboxylic acid ester
- Lactone
- Carboxylic acid derivative
- Oxacycle
- Monocarboxylic acid or derivatives
- Polyol
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organic oxygen compound
- Carbonyl group
- Organooxygen compound
- Organic oxide
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fbi-4900000000-aa407ce8b92827122435 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-00us-4009100000-3c85a877af2785527a37 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0390000000-005c046b64fa400546ea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004r-2920000000-3da2123cba34d8e561f8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-9800000000-4d0ddda730f3f457928c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0290000000-ca50e6c2cd8c6aed9f93 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00fs-2970000000-91fdf1beca5ad48fa42f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004l-7900000000-06a574a6947ca4bd6f67 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004u-1950000000-176b5d1849bbdc3eaa1f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05a9-4910000000-3bce2f7fdb3f916695a0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05o9-9600000000-dbc584e157afb6b9dbed | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-1690000000-fbe999d62045de96f6ee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f7t-8910000000-8bd6afb0fb725a6e3ec6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fk9-6900000000-a243697def0606ce40ee | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041691 |
|---|
| FooDB ID | FDB029854 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 21233624 |
|---|
| ChEBI ID | 134255 |
|---|
| PubChem Compound ID | 44389277 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. https://www.ncbi.nlm.nih.gov/pubmed/?term=11409941 | | 2. https://www.ncbi.nlm.nih.gov/pubmed/?term=12189193 | | 3. https://www.ncbi.nlm.nih.gov/pubmed/?term=18433082 | | 4. Neveu V, Perez-Jimenez J, Vos F, Crespy V, du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart D, Scalbert A: Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford). 2010;2010:bap024. doi: 10.1093/database/bap024. Epub 2010 Jan 8. |
|
|---|