| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:33:42 UTC |
|---|
| Update Date | 2016-11-09 01:22:29 UTC |
|---|
| Accession Number | CHEM041536 |
|---|
| Identification |
|---|
| Common Name | 4',7-Dihydroxy-3'-methoxyisoflavan |
|---|
| Class | Small Molecule |
|---|
| Description | 4',7-Dihydroxy-3'-methoxyisoflavan is a polyphenol metabolite detected in biological fluids (PMID: 20428313). |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
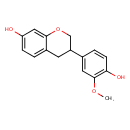
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C16H16O4 |
|---|
| Average Molecular Mass | 272.296 g/mol |
|---|
| Monoisotopic Mass | 272.105 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 3-(4-hydroxy-3-methoxyphenyl)-3,4-dihydro-2H-1-benzopyran-7-ol |
|---|
| Traditional Name | 3-(4-hydroxy-3-methoxyphenyl)-3,4-dihydro-2H-1-benzopyran-7-ol |
|---|
| SMILES | COC1=C(O)C=CC(=C1)C1COC2=CC(O)=CC=C2C1 |
|---|
| InChI Identifier | InChI=1S/C16H16O4/c1-19-16-7-10(3-5-14(16)18)12-6-11-2-4-13(17)8-15(11)20-9-12/h2-5,7-8,12,17-18H,6,9H2,1H3 |
|---|
| InChI Key | BMADVHDZKAZTNF-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 3'-o-methylated isoflavonoids. These are isoflavonoids with methoxy groups attached to the C3' atom of the isoflavonoid backbone. Isoflavonoids are natural products derived from 3-phenylchromen-4-one. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Isoflavonoids |
|---|
| Sub Class | O-methylated isoflavonoids |
|---|
| Direct Parent | 3'-O-methylated isoflavonoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 3p-methoxyisoflavonoid-skeleton
- Hydroxyisoflavonoid
- Isoflavanol
- Isoflavan
- Chromane
- Benzopyran
- Methoxyphenol
- 1-benzopyran
- Phenoxy compound
- Anisole
- Methoxybenzene
- Phenol ether
- Alkyl aryl ether
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocyclic benzene moiety
- Benzenoid
- Oxacycle
- Ether
- Organoheterocyclic compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00dr-0970000000-5ba1184f8a772aa63f79 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0ukc-6847900000-21ea77ded24a3a273671 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0960000000-3854a4fabb2c6da47aaf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0930000000-9f3237b51e09dd3c8b79 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aba-4900000000-d2653ebcd826853f22da | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0290000000-a1122cc5c82bdf519566 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0490000000-f53bf4327eda66d2601f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05fr-1930000000-f606abf5a1d199e06bb9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0090000000-e13f3bc08ebc38c670c7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05g0-0390000000-cad9678858a3dd441df9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-1790000000-57bb3e7a2bb665f6f93d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0190000000-82e54074787192cc3748 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dm-0690000000-d18724590c8fbb8bf5cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0kmr-0930000000-5c69336697710e9398d6 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041669 |
|---|
| FooDB ID | FDB029829 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 8977426 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 10802120 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Neveu V, Perez-Jimenez J, Vos F, Crespy V, du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart D, Scalbert A: Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford). 2010;2010:bap024. doi: 10.1093/database/bap024. Epub 2010 Jan 8. |
|
|---|