| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:32:32 UTC |
|---|
| Update Date | 2016-11-09 01:22:29 UTC |
|---|
| Accession Number | CHEM041493 |
|---|
| Identification |
|---|
| Common Name | 5,6-dimethyl-8-isopropenylbicyclo[4.4.0]dec-1-en-3-one |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
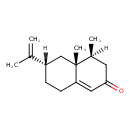
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Nootkatone | MeSH | | Nootkatone, (4R-(4alpha,4aalpha,6beta))-isomer | MeSH |
|
|---|
| Chemical Formula | C15H22O |
|---|
| Average Molecular Mass | 218.340 g/mol |
|---|
| Monoisotopic Mass | 218.167 g/mol |
|---|
| CAS Registry Number | 38427-78-0 |
|---|
| IUPAC Name | (4S,4aR,6S)-4,4a-dimethyl-6-(prop-1-en-2-yl)-2,3,4,4a,5,6,7,8-octahydronaphthalen-2-one |
|---|
| Traditional Name | (4S,4aR,6S)-4,4a-dimethyl-6-(prop-1-en-2-yl)-3,4,5,6,7,8-hexahydronaphthalen-2-one |
|---|
| SMILES | [H][C@@]1(CCC2=CC(=O)C[C@]([H])(C)[C@@]2(C)C1)C(C)=C |
|---|
| InChI Identifier | InChI=1S/C15H22O/c1-10(2)12-5-6-13-8-14(16)7-11(3)15(13,4)9-12/h8,11-12H,1,5-7,9H2,2-4H3/t11-,12-,15+/m0/s1 |
|---|
| InChI Key | WTOYNNBCKUYIKC-SLEUVZQESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as eremophilane, 8,9-secoeremophilane and furoeremophilane sesquiterpenoids. These are sesquiterpenoids with a structure based either on the eremophilane skeleton, its 8,9-seco derivative, or the furoeremophilane skeleton. Eremophilanes have been shown to be derived from eudesmanes by migration of the methyl group at C-10 to C-5. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Eremophilane, 8,9-secoeremophilane and furoeremophilane sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Eremophilane sesquiterpenoid
- Cyclohexenone
- Cyclic ketone
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0190000000-c24752b1318a3a711643 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-017i-2930000000-3df119a56dd77df5e286 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gb9-9600000000-83e236a5978c0753f062 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0090000000-985cf431add494d4b1ca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0090000000-301348660067d0dbf005 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ukm-1930000000-b1fc47101855e9adbab3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0590000000-341a117f17f53ffe7f34 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-1930000000-519467321c99880f1dba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001c-5900000000-6f03c86b1c9d475d2571 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0090000000-868e2fb00a2cfbf6c529 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0090000000-a67ecc985c1a2f05f9c1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-015a-0930000000-7dec12d146ded38aa03b | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0303909 |
|---|
| FooDB ID | FDB029762 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 5884295 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 7567181 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|