| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:28:22 UTC |
|---|
| Update Date | 2016-11-09 01:22:28 UTC |
|---|
| Accession Number | CHEM041411 |
|---|
| Identification |
|---|
| Common Name | O-Arachidonoyl ethanolamine |
|---|
| Class | Small Molecule |
|---|
| Description | Arachidonoyl ethanolamide (AEA) was the first endogenous cannabinoid to be isolated and characterized as an agonist acting on the same receptors (CB1 and CB2) as tetrahydrocannabinols (THC). Since that time, a number of related endocannabinoids have been isolated, most notably 2-arachidonoyl glycerol (2-AG).O-Arachidonoyl ethanolamine hydrochloride (O-AEA) is a recently isolated constituent of human and rat brain wherein the ethanolamine moiety is attached "backwards", as an ester instead of an amide, as in AEA.1,2,4 O-AEA has mixed agonist/antagonist activity at the CB1 receptor and does not appear to be the native endogenous cannabinoid agonist at this receptor. This is in keeping with other observations that 2-AG is the primary endogenous CB1 receptor ligand. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
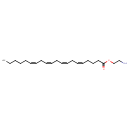
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Arachidonic acid-(2-aminoethyl)-ester | HMDB | | O-AEA | HMDB | | Virodhamine | HMDB | | O-Arachidonyl ethanol amine | MeSH, HMDB | | O-Arachidonoyl ethanolamine | MeSH |
|
|---|
| Chemical Formula | C22H37NO2 |
|---|
| Average Molecular Mass | 347.535 g/mol |
|---|
| Monoisotopic Mass | 347.282 g/mol |
|---|
| CAS Registry Number | 443129-35-9 |
|---|
| IUPAC Name | 2-aminoethyl (5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoate |
|---|
| Traditional Name | virodhamine |
|---|
| SMILES | CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)OCCN |
|---|
| InChI Identifier | InChI=1S/C22H37NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-22(24)25-21-20-23/h6-7,9-10,12-13,15-16H,2-5,8,11,14,17-21,23H2,1H3/b7-6-,10-9-,13-12-,16-15- |
|---|
| InChI Key | DLHLOYYQQGSXCC-DOFZRALJSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as fatty acid esters. These are carboxylic ester derivatives of a fatty acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acid esters |
|---|
| Direct Parent | Fatty acid esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Fatty acid ester
- Amino acid or derivatives
- Carboxylic acid ester
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Amine
- Primary aliphatic amine
- Organopnictogen compound
- Organic oxygen compound
- Carbonyl group
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-9160000000-e4d605556a74c32850b5 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0005-6159000000-99134b0a76bda27146bc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9241000000-24b121d519022021b041 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9230000000-34e0714a863ac0a11fe4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000b-2049000000-44ec5ce62678fce7c991 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000j-5097000000-226c63be9164349ed9bf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9030000000-f393ed4f36b8e090cc1a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-4329000000-2afbe38e34d21b7d29ed | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01po-9412000000-2d595dbad447acf1e266 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00l6-9500000000-37b7a889796f7361f97e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0029000000-6d820b2c37cd6bf13b7e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f7a-5189000000-c237f01fc5e28353b369 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uk9-9385000000-ece22a403f9d3abaf5f1 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0013655 |
|---|
| FooDB ID | FDB029625 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Virodhamine |
|---|
| Chemspider ID | 4650158 |
|---|
| ChEBI ID | 418207 |
|---|
| PubChem Compound ID | 5712057 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|