| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:17:21 UTC |
|---|
| Update Date | 2016-11-09 01:22:26 UTC |
|---|
| Accession Number | CHEM041233 |
|---|
| Identification |
|---|
| Common Name | PC(o-18:2(9Z,12Z)/22:0) |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
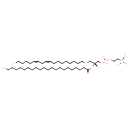
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Linoleyl-2-behenoyl-sn-glycero-3-phosphocholine | HMDB | | Gpcho(18:2/22:0) | HMDB | | Gpcho(18:2n6/22:0) | HMDB | | Gpcho(18:2W6/22:0) | HMDB | | Gpcho(40:2) | HMDB | | Lecithin | HMDB | | PC Ae C40:2 | HMDB | | PC(18:2/22:0) | HMDB | | PC(18:2n6/22:0) | HMDB | | PC(18:2W6/22:0) | HMDB | | PC(40:2) | HMDB | | PC(O-40:2) | HMDB | | Phosphatidylcholine(18:2/22:0) | HMDB | | Phosphatidylcholine(18:2n6/22:0) | HMDB | | Phosphatidylcholine(18:2W6/22:0) | HMDB | | Phosphatidylcholine(40:2) | HMDB | | 1-(9Z,12Z-Octadecadienyl)-2-docosanoyl-sn-glycero-3-phosphocholine | HMDB | | PC(o-18:2(9Z,12Z)/22:0) | Lipid Annotator |
|
|---|
| Chemical Formula | C48H94NO7P |
|---|
| Average Molecular Mass | 828.236 g/mol |
|---|
| Monoisotopic Mass | 827.677 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (2-{[(2R)-2-(docosanoyloxy)-3-[(9Z,12Z)-octadeca-9,12-dien-1-yloxy]propyl phosphonato]oxy}ethyl)trimethylazanium |
|---|
| Traditional Name | (2-{[(2R)-2-(docosanoyloxy)-3-[(9Z,12Z)-octadeca-9,12-dien-1-yloxy]propyl phosphonato]oxy}ethyl)trimethylazanium |
|---|
| SMILES | CCCCCCCCCCCCCCCCCCCCCC(=O)O[C@]([H])(COCCCCCCCC\C=C/C\C=C/CCCCC)COP([O-])(=O)OCC[N+](C)(C)C |
|---|
| InChI Identifier | InChI=1S/C48H94NO7P/c1-6-8-10-12-14-16-18-20-22-24-25-26-27-29-31-33-35-37-39-41-48(50)56-47(46-55-57(51,52)54-44-42-49(3,4)5)45-53-43-40-38-36-34-32-30-28-23-21-19-17-15-13-11-9-7-2/h15,17,21,23,47H,6-14,16,18-20,22,24-46H2,1-5H3/b17-15-,23-21-/t47-/m1/s1 |
|---|
| InChI Key | DMPIMBNFIDOLGO-DTRJYZOJSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1-alkyl,2-acylglycero-3-phosphocholines. These are glycerophosphocholines that carry exactly one acyl chain attached to the glycerol moiety through an ester linkage at the O2-position, and one alkyl chain attached through an ether linkage at the O1-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerophospholipids |
|---|
| Sub Class | Glycerophosphocholines |
|---|
| Direct Parent | 1-alkyl,2-acylglycero-3-phosphocholines |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1-alkyl,2-acylglycero-3-phosphocholine
- Phosphocholine
- Fatty acid ester
- Dialkyl phosphate
- Glycerol ether
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Fatty acyl
- Alkyl phosphate
- Tetraalkylammonium salt
- Quaternary ammonium salt
- Carboxylic acid ester
- Carboxylic acid derivative
- Dialkyl ether
- Ether
- Monocarboxylic acid or derivatives
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Carbonyl group
- Amine
- Organic nitrogen compound
- Organooxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organic salt
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-9105212130-836491e9990df5da77f1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05a2-6349112210-6484998bc2276a82a57a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ei-9057003200-400d8d159ca45ba6eb32 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004r-0027000390-86a950e6f9a4fcfface2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0109-2076200920-1c69577f7393d530fbfd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00tv-7069200000-9bcca19ef5e7180b6a71 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000000090-808d4510c97d542db5e0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0013002290-9cd90a346253d8cd9608 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kr-5019800000-fc64860dd6bb2fa2e489 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000000090-e58ae67343e2207d0987 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0000000090-e58ae67343e2207d0987 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udr-0006300190-7c3a8c2f48bc27f8edd7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0000000090-caf59510d6707c21da94 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0000000090-caf59510d6707c21da94 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-003r-1900141160-d0321232ae0d4b1fa2c8 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0013437 |
|---|
| FooDB ID | FDB029437 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 89868 |
|---|
| PubChem Compound ID | 53481739 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|