| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:15:02 UTC |
|---|
| Update Date | 2016-11-09 01:22:24 UTC |
|---|
| Accession Number | CHEM041132 |
|---|
| Identification |
|---|
| Common Name | 5-Androstene-3alpha,16beta,17beta-triol |
|---|
| Class | Small Molecule |
|---|
| Description | A human metabolite taken as a putative food compound of mammalian origin |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
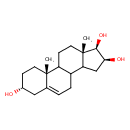
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5-Androstene-3a-16b,17b-triol | Generator | | 5-Androstene-3α-16b,17b-triol | Generator |
|
|---|
| Chemical Formula | C19H30O3 |
|---|
| Average Molecular Mass | 306.440 g/mol |
|---|
| Monoisotopic Mass | 306.219 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (2R,5R,13S,14R,15S)-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-ene-5,13,14-triol |
|---|
| Traditional Name | (2R,5R,13S,14R,15S)-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-ene-5,13,14-triol |
|---|
| SMILES | C[C@]12CCC3C(CC=C4C[C@H](O)CC[C@]34C)C1C[C@H](O)[C@@H]2O |
|---|
| InChI Identifier | InChI=1S/C19H30O3/c1-18-7-5-12(20)9-11(18)3-4-13-14(18)6-8-19(2)15(13)10-16(21)17(19)22/h3,12-17,20-22H,4-10H2,1-2H3/t12-,13?,14?,15?,16+,17+,18+,19+/m1/s1 |
|---|
| InChI Key | GUGSXATYPSGVAY-YAUITSCLSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as androgens and derivatives. These are 3-hydroxylated C19 steroid hormones. They are known to favor the development of masculine characteristics. They also show profound effects on scalp and body hair in humans. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Androstane steroids |
|---|
| Direct Parent | Androgens and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Androgen-skeleton
- 3-hydroxy-delta-5-steroid
- 3-hydroxysteroid
- Hydroxysteroid
- 16-beta-hydroxysteroid
- 16-hydroxysteroid
- 3-alpha-hydroxysteroid
- 17-hydroxysteroid
- Delta-5-steroid
- Cyclic alcohol
- Secondary alcohol
- Polyol
- Organooxygen compound
- Alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03fr-0090000000-4e39e270f360af16787f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0a4i-1013980000-19f683f12fe72ee4fbe8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-0094000000-fd24b7644323a9a8e64a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0079-0191000000-ee6440f64ac2ade69d3b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-022c-3690000000-93ef29ee24b8ab4da986 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0039000000-575557def76fdbc5c613 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4r-0089000000-bde6dc328afc8b9ae9c8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-059i-0090000000-14b0f3cc88c65449e485 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0013199 |
|---|
| FooDB ID | FDB029327 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 53481647 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|