| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:08:15 UTC |
|---|
| Update Date | 2016-11-09 01:22:23 UTC |
|---|
| Accession Number | CHEM041021 |
|---|
| Identification |
|---|
| Common Name | Arginine vasopressin 1-8 |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
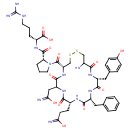
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Cys-tyr-phe-GLN-asn-cys-pro-arg | HMDB | | Des-gly-NH(,2)('9),[arg('8)]-vasopressin | HMDB | | (2R)-2-({[(2R)-1-[(4R,10R,13R,16R)-19-amino-13-benzyl-6,9,12,15,18-pentahydroxy-10-[2-(C-hydroxycarbonimidoyl)ethyl]-7-[(C-hydroxycarbonimidoyl)methyl]-16-[(4-hydroxyphenyl)methyl]-1,2-dithia-5,8,11,14,17-pentaazacycloicosa-5,8,11,14,17-pentaene-4-carbonyl]pyrrolidin-2-yl](hydroxy)methylidene}amino)-5-carbamimidamidopentanoate | Generator |
|
|---|
| Chemical Formula | C44H61N13O12S2 |
|---|
| Average Molecular Mass | 1028.165 g/mol |
|---|
| Monoisotopic Mass | 1027.400 g/mol |
|---|
| CAS Registry Number | 37552-33-3 |
|---|
| IUPAC Name | (2R)-2-{[(2R)-1-[(4R,10R,13R,16R)-19-amino-13-benzyl-10-[2-(C-hydroxycarbonimidoyl)ethyl]-7-[(C-hydroxycarbonimidoyl)methyl]-16-[(4-hydroxyphenyl)methyl]-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaazacycloicosane-4-carbonyl]pyrrolidin-2-yl]formamido}-5-carbamimidamidopentanoic acid |
|---|
| Traditional Name | (2R)-2-{[(2R)-1-[(4R,10R,13R,16R)-19-amino-13-benzyl-10-[2-(C-hydroxycarbonimidoyl)ethyl]-7-(C-hydroxycarbonimidoylmethyl)-16-[(4-hydroxyphenyl)methyl]-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaazacycloicosane-4-carbonyl]pyrrolidin-2-yl]formamido}-5-carbamimidamidopentanoic acid |
|---|
| SMILES | NC1CSSC[C@H](NC(=O)C(CC(O)=N)NC(=O)[C@@H](CCC(O)=N)NC(=O)[C@@H](CC2=CC=CC=C2)NC(=O)[C@@H](CC2=CC=C(O)C=C2)NC1=O)C(=O)N1CCC[C@@H]1C(=O)N[C@H](CCCNC(N)=N)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C44H61N13O12S2/c45-26-21-70-71-22-32(42(67)57-17-5-9-33(57)41(66)52-28(43(68)69)8-4-16-50-44(48)49)56-40(65)31(20-35(47)60)55-37(62)27(14-15-34(46)59)51-38(63)30(18-23-6-2-1-3-7-23)54-39(64)29(53-36(26)61)19-24-10-12-25(58)13-11-24/h1-3,6-7,10-13,26-33,58H,4-5,8-9,14-22,45H2,(H2,46,59)(H2,47,60)(H,51,63)(H,52,66)(H,53,61)(H,54,64)(H,55,62)(H,56,65)(H,68,69)(H4,48,49,50)/t26?,27-,28-,29-,30-,31?,32+,33-/m1/s1 |
|---|
| InChI Key | SXYIOPJBWYQZRQ-VMPYSSHJSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as long-chain 3-enoyl coas. These are organic compounds containing a coenzyme A substructure linked to a long-chain 3-enoyl chain of 13 to 21 carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acyl thioesters |
|---|
| Direct Parent | Long-chain 3-enoyl CoAs |
|---|
| Alternative Parents | |
|---|
| Substituents | - Coenzyme a or derivatives
- Purine ribonucleoside 3',5'-bisphosphate
- Purine ribonucleoside bisphosphate
- Purine ribonucleoside diphosphate
- Ribonucleoside 3'-phosphate
- Pentose phosphate
- Pentose-5-phosphate
- Octadecanoid
- Long-chain fatty acid
- Cysteine or derivatives
- Glycosyl compound
- N-glycosyl compound
- Alpha-amino acid
- Alpha-amino acid or derivatives
- 6-aminopurine
- Monosaccharide phosphate
- Organic pyrophosphate
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Aminopyrimidine
- Hydroxy fatty acid
- Monoalkyl phosphate
- Thia fatty acid
- Fatty acid
- Imidolactam
- Dicarboxylic acid or derivatives
- Alkyl phosphate
- Monosaccharide
- N-substituted imidazole
- Organic phosphoric acid derivative
- Unsaturated fatty acid
- Phosphoric acid ester
- Pyrimidine
- Imidazole
- Oxolane
- Azole
- Heteroaromatic compound
- Amino acid
- Secondary alcohol
- Thiocarboxylic acid ester
- Carbothioic s-ester
- Amino acid or derivatives
- Dialkylthioether
- Sulfenyl compound
- Oxacycle
- Thioether
- Carboximidic acid
- Azacycle
- Thiocarboxylic acid or derivatives
- Propargyl-type 1,3-dipolar organic compound
- Carboximidic acid derivative
- Organic 1,3-dipolar compound
- Organoheterocyclic compound
- Carboxylic acid derivative
- Carboxylic acid
- Hydrocarbon derivative
- Organic nitrogen compound
- Alcohol
- Organic oxygen compound
- Organic oxide
- Primary aliphatic amine
- Primary amine
- Amine
- Organonitrogen compound
- Organooxygen compound
- Carbonyl group
- Organosulfur compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-9110000025-1d304e1782b2b9291198 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03fr-9410000648-470eae3d17a7f7d705bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0229-9100000100-4b40efe5af3314f71a4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a7i-8000000009-b503dd2d74647ca7d085 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4l-9110000017-bd06143bf9064ccc775e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9100000000-4e293036f769e50bf0f8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-9000000001-b63902d3b4c23aa08ec7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05mo-6100000009-7777e6244da2676c85ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000010-2cc8a7f561fd1c804ae0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01t9-9000000020-ae807e7e466480b191d6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03e9-9010000315-5e0e5a761015a74ef4fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01x3-6200000930-54c5785551341cad6b32 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0012895 |
|---|
| FooDB ID | FDB029201 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35032552 |
|---|
| ChEBI ID | 89492 |
|---|
| PubChem Compound ID | 53481539 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|