| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:07:38 UTC |
|---|
| Update Date | 2016-11-09 01:22:22 UTC |
|---|
| Accession Number | CHEM040996 |
|---|
| Identification |
|---|
| Common Name | 4-Oxoretinal |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
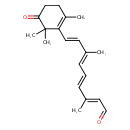
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,7-Dimethyl-9-(5-oxo-2,6,6-trimethyl-1-cyclohexenyl)-all-trans-2,4,6,8-nonatetraenal | HMDB | | 4-Ketoretinal | HMDB | | 4-oxo-all-trans-Retinal | HMDB | | 4OVA | HMDB |
|
|---|
| Chemical Formula | C20H26O2 |
|---|
| Average Molecular Mass | 298.419 g/mol |
|---|
| Monoisotopic Mass | 298.193 g/mol |
|---|
| CAS Registry Number | 33532-44-4 |
|---|
| IUPAC Name | (2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethyl-5-oxocyclohex-1-en-1-yl)nona-2,4,6,8-tetraenal |
|---|
| Traditional Name | (2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethyl-5-oxocyclohex-1-en-1-yl)nona-2,4,6,8-tetraenal |
|---|
| SMILES | C\C(\C=C\C=C(/C)\C=C\C1=C(C)CCC(=O)C1(C)C)=C/C=O |
|---|
| InChI Identifier | InChI=1S/C20H26O2/c1-15(7-6-8-16(2)13-14-21)9-11-18-17(3)10-12-19(22)20(18,4)5/h6-9,11,13-14H,10,12H2,1-5H3/b8-6+,11-9+,15-7+,16-13+ |
|---|
| InChI Key | ZORDCCAUKPDLPN-ZBSJWCJSSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as retinoids. These are oxygenated derivatives of 3,7-dimethyl-1-(2,6,6-trimethylcyclohex-1-enyl)nona-1,3,5,7-tetraene and derivatives thereof. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Retinoids |
|---|
| Direct Parent | Retinoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Retinoid skeleton
- Diterpenoid
- Cyclohexenone
- Enal
- Alpha,beta-unsaturated aldehyde
- Cyclic ketone
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aldehyde
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-053r-2390000000-ae80440018b7880e093d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0490000000-a6823593099fbaed6c4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-2960000000-3a4e5a096946e2cb9d53 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f92-7940000000-6d7cc7a2009a0f4145a2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-31e800acd103df7baca2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014j-0090000000-8bed0f51ae47dee8402f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0gbc-5390000000-900d357ffb0b666f354e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014j-0090000000-733fa43232ceb2888d4f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0190000000-3a1a823f6ec06c3927c2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-1390000000-0d264f4d7434c6f8f7ea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001a-0970000000-de4c95545bd13a24b1dd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0bu9-1980000000-12284bfbc8b14f433bf7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00n3-7910000000-7841e7f00250ad2177d4 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0012794 |
|---|
| FooDB ID | FDB029176 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30776653 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 53481523 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|