| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:05:30 UTC |
|---|
| Update Date | 2016-11-09 01:22:21 UTC |
|---|
| Accession Number | CHEM040950 |
|---|
| Identification |
|---|
| Common Name | 13'-Hydroxy-gamma-tocopherol |
|---|
| Class | Small Molecule |
|---|
| Description | 13'-hydroxy-r-tocopherol is a precursor of dehydrogenation to form 13'-Carboxy-gamma-tocopherol by an unidentified microsomal enzyme(s) probably via an aldehyde intermediate. r-Tocopherol provides different antioxidant activities in food and in-vitro studies and showed higher activity in trapping lipophilic electrophiles and reactive nitrogen and oxygen species. From the metabolism end product, only that of r-tocopherol (2,7,8-trimethyl-2-(b-carboxyethyl)-6-hydroxychroman), but not that of a-tocopherol, was identified to provide natriuretic activity. Only the r-tocopherol plasma level served as biomarker for cancer and cardiovascular risk. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
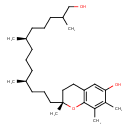
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 13'-Hydroxy-g-tocopherol | Generator | | 13'-Hydroxy-γ-tocopherol | Generator | | 13'-OH-Tocopherol | HMDB |
|
|---|
| Chemical Formula | C28H48O3 |
|---|
| Average Molecular Mass | 432.679 g/mol |
|---|
| Monoisotopic Mass | 432.360 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (2R)-2-[(4S,8R)-13-hydroxy-4,8,12-trimethyltridecyl]-2,7,8-trimethyl-3,4-dihydro-2H-1-benzopyran-6-ol |
|---|
| Traditional Name | (2R)-2-[(4S,8R)-13-hydroxy-4,8,12-trimethyltridecyl]-2,7,8-trimethyl-3,4-dihydro-1-benzopyran-6-ol |
|---|
| SMILES | CC(CO)CCC[C@H](C)CCC[C@H](C)CCC[C@]1(C)CCC2=C(O1)C(C)=C(C)C(O)=C2 |
|---|
| InChI Identifier | InChI=1S/C28H48O3/c1-20(12-8-13-22(3)19-29)10-7-11-21(2)14-9-16-28(6)17-15-25-18-26(30)23(4)24(5)27(25)31-28/h18,20-22,29-30H,7-17,19H2,1-6H3/t20-,21+,22?,28-/m1/s1 |
|---|
| InChI Key | QNBPVJMQPAQXML-SLGTZZLGSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tocopherols. These are vitamin E derivatives containing a saturated trimethyltridecyl chain attached to the carbon C6 atom of a benzopyran ring system. The differ from tocotrienols that contain an unsaturated trimethyltrideca-3,7,11-trien-1-yl chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Quinone and hydroquinone lipids |
|---|
| Direct Parent | Tocopherols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tocopherol
- Diterpenoid
- Long chain fatty alcohol
- Chromane
- Benzopyran
- 1-benzopyran
- Fatty alcohol
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Fatty acyl
- Benzenoid
- Organoheterocyclic compound
- Ether
- Oxacycle
- Organic oxygen compound
- Alcohol
- Hydrocarbon derivative
- Organooxygen compound
- Primary alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-03di-1454390000-7842b0b1bb538653ff9c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0hnf-2975400000-7bb7c14065fa1f987a6f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fsi-0421900000-f32e1218cd4b25480728 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0921100000-9af955e3a6ca5cc9b504 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-2910000000-b9fac219f3c4d2615086 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0000900000-b973ab3c74e8641e9199 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01qa-0501900000-7a7396d4bce194c6cd9a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000j-2926300000-eaf0c2bb9bb0a46b4cee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01q9-0000900000-93b97149c3e76cb52886 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0gx1-0302900000-814090875d1ef4bed12b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03kd-0809500000-19adb2966ed984243e6b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-2224900000-f43893fdf5951a6996f9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000b-7927100000-0509051ba7c4b3d9aeba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05tg-7910000000-c37a1015fcc54a9911a4 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0012561 |
|---|
| FooDB ID | FDB029127 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35032541 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 53481467 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|