| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:05:13 UTC |
|---|
| Update Date | 2016-11-09 01:22:21 UTC |
|---|
| Accession Number | CHEM040936 |
|---|
| Identification |
|---|
| Common Name | 11beta-Hydroxytestosterone |
|---|
| Class | Small Molecule |
|---|
| Description | 11beta-Hydroxytestosterone is a Testosterone derivative metabolite. Testosterone is reported to have an acute vasodilating action in vitro, an effect that may impart a favourable haemodynamic response in patients with chronic heart failure. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
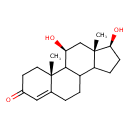
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 11b-Hydroxytestosterone | Generator | | 11Β-hydroxytestosterone | Generator | | 11beta,17beta-Dihydroxyandrost-4-en-3-one | HMDB |
|
|---|
| Chemical Formula | C19H28O3 |
|---|
| Average Molecular Mass | 304.424 g/mol |
|---|
| Monoisotopic Mass | 304.204 g/mol |
|---|
| CAS Registry Number | 1816-85-9 |
|---|
| IUPAC Name | (2R,14S,15S,17S)-14,17-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-6-en-5-one |
|---|
| Traditional Name | (2R,14S,15S,17S)-14,17-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-6-en-5-one |
|---|
| SMILES | C[C@]12C[C@H](O)C3C(CCC4=CC(=O)CC[C@]34C)C1CC[C@@H]2O |
|---|
| InChI Identifier | InChI=1S/C19H28O3/c1-18-8-7-12(20)9-11(18)3-4-13-14-5-6-16(22)19(14,2)10-15(21)17(13)18/h9,13-17,21-22H,3-8,10H2,1-2H3/t13?,14?,15-,16-,17?,18-,19-/m0/s1 |
|---|
| InChI Key | YQDZGFAYWGWSJK-UALLODJUSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as androgens and derivatives. These are 3-hydroxylated C19 steroid hormones. They are known to favor the development of masculine characteristics. They also show profound effects on scalp and body hair in humans. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Androstane steroids |
|---|
| Direct Parent | Androgens and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Androgen-skeleton
- 3-oxo-delta-4-steroid
- 3-oxosteroid
- 17-hydroxysteroid
- 11-hydroxysteroid
- 11-beta-hydroxysteroid
- Oxosteroid
- Hydroxysteroid
- Delta-4-steroid
- Cyclohexenone
- Cyclic alcohol
- Cyclic ketone
- Secondary alcohol
- Ketone
- Organic oxygen compound
- Organooxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Alcohol
- Organic oxide
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a70-1490000000-dc3a8637553ec30a81af | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-001i-6416900000-47f5e5efe9ebc3c0c60c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-0092000000-d9d4c1c317457f2cd8b1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ap0-0190000000-4c7bbe3784b787407369 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aor-4290000000-12d89219e86f42485bd3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0049000000-636215ca584ee024fa71 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udr-0098000000-a05c39e18149db0aee36 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05g3-2090000000-3f14dd2657cd68ffee03 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0079000000-cf676a386df1d37c40ef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-02ti-1490000000-5fbd372576f2bfb7fbf6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ox-7940000000-6609b365d4f5ace80acb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0009000000-93986d59045cc7fce551 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0009000000-dc144522dc3fe4299e0a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-0297000000-3915f43c121e8b1e71d5 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0012533 |
|---|
| FooDB ID | FDB029113 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 7993705 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 9817955 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|