| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:05:08 UTC |
|---|
| Update Date | 2016-11-09 01:22:21 UTC |
|---|
| Accession Number | CHEM040933 |
|---|
| Identification |
|---|
| Common Name | 11'-Carboxy-gamma-chromanol |
|---|
| Class | Small Molecule |
|---|
| Description | 11'-Carboxy-gamma-tocopherol is a dehydrogenation carboxylate product of 11'-hydroxy-r-tocopherol by an unidentified microsomal enzyme(s) probably via an aldehyde intermediate. r-Tocopherol provides different antioxidant activities in food and in-vitro studies and showed higher activity in trapping lipophilic electrophiles and reactive nitrogen and oxygen species. From the metabolism end product, only that of r-tocopherol (2,7,8-trimethyl-2-(b-carboxyethyl)-6-hydroxychroman), but not that of a-tocopherol, was identified to provide natriuretic activity. Only the r-tocopherol plasma level served as biomarker for cancer and cardiovascular risk. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
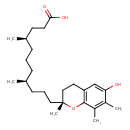
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 11'-Carboxy-gamma-tocopherol | HMDB | | (4R,8S)-11-[(2R)-6-Hydroxy-2,7,8-trimethyl-3,4-dihydro-2H-1-benzopyran-2-yl]-4,8-dimethylundecanoate | Generator |
|
|---|
| Chemical Formula | C25H40O4 |
|---|
| Average Molecular Mass | 404.583 g/mol |
|---|
| Monoisotopic Mass | 404.293 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (4R,8S)-11-[(2R)-6-hydroxy-2,7,8-trimethyl-3,4-dihydro-2H-1-benzopyran-2-yl]-4,8-dimethylundecanoic acid |
|---|
| Traditional Name | (4R,8S)-11-[(2R)-6-hydroxy-2,7,8-trimethyl-3,4-dihydro-1-benzopyran-2-yl]-4,8-dimethylundecanoic acid |
|---|
| SMILES | C[C@@H](CCC[C@@H](C)CCC(O)=O)CCC[C@]1(C)CCC2=C(O1)C(C)=C(C)C(O)=C2 |
|---|
| InChI Identifier | InChI=1S/C25H40O4/c1-17(8-6-9-18(2)11-12-23(27)28)10-7-14-25(5)15-13-21-16-22(26)19(3)20(4)24(21)29-25/h16-18,26H,6-15H2,1-5H3,(H,27,28)/t17-,18+,25+/m0/s1 |
|---|
| InChI Key | IITULCXNOMOXAH-YYULODDRSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sesquiterpenoids. These are terpenes with three consecutive isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sesquiterpenoid
- Long-chain fatty acid
- Chromane
- Benzopyran
- 1-benzopyran
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Branched fatty acid
- Heterocyclic fatty acid
- Methyl-branched fatty acid
- Hydroxy fatty acid
- Fatty acyl
- Fatty acid
- Benzenoid
- Oxacycle
- Carboxylic acid derivative
- Carboxylic acid
- Ether
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Organic oxide
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Carbonyl group
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01px-3789000000-b9cb7dc2edc9c0dc3149 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-001i-4388590000-bdc6d60eb866d7a8b7ef | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-0529200000-08553bc979f15487e787 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0911000000-9e630eaf829f03921b29 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-1910000000-f8b7aad184832fe318dc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0103900000-dda02cca2ec6a1b6c1cc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0k9b-2719500000-fa011191e66ab5e1d820 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4v-9715000000-067f230061e510e29e79 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0zfr-0007900000-c691010f380fd137af49 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4l-6209300000-6d88fde92393398bc7d4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006x-1913000000-242862c102afa2200c76 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0139000000-4a4c307e44e7b68aa3a3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a5d-2492000000-d883fbb7be140d78fd9c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-006x-3920000000-54856887377167d10023 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0012517 |
|---|
| FooDB ID | FDB029110 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30776628 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 53481453 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|