| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:05:02 UTC |
|---|
| Update Date | 2016-11-09 01:22:21 UTC |
|---|
| Accession Number | CHEM040928 |
|---|
| Identification |
|---|
| Common Name | 10,11-Dihydro-20-trihydroxy-leukotriene B4 |
|---|
| Class | Small Molecule |
|---|
| Description | 10,11-dihydro-20-trihydroxy-leukotriene B4 is the metabolite of lipid omega-oxidation of leukotriene B4 (LTB4). LTB4 is the major metabolite in neutrophil polymorphonuclear leukocytes. Omega-oxidation is the major pathway for the catabolism of leukotriene B4 in human polymorphonuclear leukocytes. Leukotrienes are metabolites of arachidonic acid derived from the action of 5-LO (5-lipoxygenase). The immediate product of 5-LO is LTA4 (leukotriene A4), which is enzymatically converted into either LTB4 (leukotriene B4) by LTA4 hydrolase or LTC4 (leukotriene C4) by LTC4 synthase. The regulation of leukotriene production occurs at various levels, including expression of 5-LO, translocation of 5-LO to the perinuclear region, and phosphorylation to either enhance or inhibit the activity of 5-LO. Biologically active LTB4 is metabolized by omega-oxidation carried out by specific cytochrome P450s (CYP4F) followed by beta-oxidation from the omega-carboxy position and after CoA ester formation (PMID: 7649996, 17623009, 2853166, 6088485). Leukotrienes are eicosanoids. The eicosanoids consist of the prostaglandins (PGs), thromboxanes (TXs), leukotrienes (LTs), and lipoxins (LXs). The PGs and TXs are collectively identified as prostanoids. Prostaglandins were originally shown to be synthesized in the prostate gland, thromboxanes from platelets (thrombocytes), and leukotrienes from leukocytes, hence the derivation of their names. All mammalian cells except erythrocytes synthesize eicosanoids. These molecules are extremely potent, able to cause profound physiological effects at very dilute concentrations. All eicosanoids function locally at the site of synthesis, through receptor-mediated G-protein linked signalling pathways. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
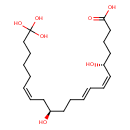
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (5S,12R,20)-Pentahydroxy-(6Z,8E,14Z)-eicosatrienoate | HMDB | | (5S,12R,20)-Pentahydroxy-(6Z,8E,14Z)-eicosatrienoic acid | HMDB | | (5S,12R,20)-Pentahydroxy-(6Z,8E,14Z)-eicosatrienoic acid anion | HMDB |
|
|---|
| Chemical Formula | C20H34O7 |
|---|
| Average Molecular Mass | 386.480 g/mol |
|---|
| Monoisotopic Mass | 386.230 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (5R,6Z,8E,12R,14Z)-5,12,20,20,20-pentahydroxyicosa-6,8,14-trienoic acid |
|---|
| Traditional Name | (5R,6Z,8E,12R,14Z)-5,12,20,20,20-pentahydroxyicosa-6,8,14-trienoic acid |
|---|
| SMILES | O[C@H](CC\C=C\C=C/[C@H](O)CCCC(O)=O)C\C=C/CCCCC(O)(O)O |
|---|
| InChI Identifier | InChI=1S/C20H34O7/c21-17(11-6-2-1-5-9-16-20(25,26)27)12-7-3-4-8-13-18(22)14-10-15-19(23)24/h2-4,6,8,13,17-18,21-22,25-27H,1,5,7,9-12,14-16H2,(H,23,24)/b4-3+,6-2-,13-8-/t17-,18-/m0/s1 |
|---|
| InChI Key | LSVHZFZMMXHHBS-MNBASXMYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydroxyeicosatrienoic acids. These are eicosanoic acids with an attached hydroxyl group and three CC double bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Eicosanoids |
|---|
| Direct Parent | Hydroxyeicosatrienoic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydroxyeicosatrienoic acid
- Long-chain fatty acid
- Hydroxy fatty acid
- Fatty acid
- Unsaturated fatty acid
- Secondary alcohol
- Ortho acid
- Orthocarboxylic acid derivative
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Polyol
- Organic oxide
- Organic oxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organooxygen compound
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014i-4976000000-58dafb37ebfe673dc4d7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0a4i-3373159000-5af552949a2907d3cddb | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uxr-0009000000-03b13e62a3637e396f0e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-106r-0129000000-4bdb31c9a796f9561084 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-06r7-6395000000-448adcec0fd216b9bc17 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00kr-0009000000-9e7df281c4954357313a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-06dr-1009000000-97c7b75aae8eb95347ec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a6r-9122000000-92ac0fbec264bac85376 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014r-0009000000-6101fa129947137f2348 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-6119000000-0590c80a59036ea8c398 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05mk-6159000000-1b04c27a75b4f579375d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uxr-0019000000-0523643e990562259eff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0097000000-9c1ea28da410fde70add | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-4890000000-b5a4aaefab1f1296704f | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0012503 |
|---|
| FooDB ID | FDB029105 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30776623 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 53481448 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|