| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:04:45 UTC |
|---|
| Update Date | 2016-11-09 01:22:21 UTC |
|---|
| Accession Number | CHEM040920 |
|---|
| Identification |
|---|
| Common Name | 1,2-Dehydrosalsolinol |
|---|
| Class | Small Molecule |
|---|
| Description | 1,2-dehydrosalsolinol(1-methyl-6,7-dihydroxy-3,4-dihydroisoquinolines) is formed through the decarboxylation of salsolinol-1-carboxylic acid (1-methyl-6,7-dihydroxy-1,2,3,4- tetrahydroisoquinoline-1-carboxylic acid), a novel endogenous catecholic adduct of dopamine and pyruvic acid, examined in nuclei-free homogenates of rat liver, whole brain, and kidney, as well as in buffer only. Liquid chromatographic analysis of incubations for varying times (30 min to 5 h) showed that the tetrahydroisoquinoline substrate decarboxylated oxidatively, forming the DSAL (PMID: 3369867). It is involved in Tyrosine Metabolism. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
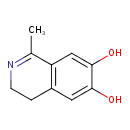
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Methyl-6,7-dihydroxy-3,4-dihydroisoquinolines | HMDB | | 3,4-dihydro-1-Methyl-6,7-isoquinolinediol | HMDB | | DSAL | HMDB |
|
|---|
| Chemical Formula | C10H11NO2 |
|---|
| Average Molecular Mass | 177.200 g/mol |
|---|
| Monoisotopic Mass | 177.079 g/mol |
|---|
| CAS Registry Number | 4602-81-7 |
|---|
| IUPAC Name | 1-methyl-3,4-dihydroisoquinoline-6,7-diol |
|---|
| Traditional Name | 1-methyl-3,4-dihydroisoquinoline-6,7-diol |
|---|
| SMILES | CC1=NCCC2=C1C=C(O)C(O)=C2 |
|---|
| InChI Identifier | InChI=1S/C10H11NO2/c1-6-8-5-10(13)9(12)4-7(8)2-3-11-6/h4-5,12-13H,2-3H2,1H3 |
|---|
| InChI Key | DWHSGRLKLUGKOU-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dihydroisoquinolines. These are isoquinoline derivatives where exactly two carbon atoms joined by an aromatic bond are linked with a hydrogen atom each, resulting in a CC single bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Dihydroisoquinolines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Dihydroisoquinolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dihydroisoquinoline
- 1-hydroxy-2-unsubstituted benzenoid
- Benzenoid
- Ketimine
- Azacycle
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Imine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-05fr-1392000000-f1e4a0be2f7e97323ee6 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01ot-0900000000-12d9d22ce9d1f53763a2 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0900000000-3353063db7785c56ab4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002r-1900000000-60a5f8487936dabfac02 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00vr-7900000000-9a57ee165717efdad1c8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0900000000-f972bf5f261cc77d06c0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0900000000-7529c280110b6380588a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-07w9-1900000000-3d8656d3388a0f99e63e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0900000000-ef6ae59e91e658cf298a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0900000000-ce3e229270093bb2c74b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00xr-1900000000-f91c36aaafb986741bf5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0900000000-287b3d60ee7ac7576fb4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0900000000-5def6855fd9d71e1370e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-4900000000-e69f269d4d26d100a113 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0012490 |
|---|
| FooDB ID | FDB029097 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 17960720 |
|---|
| ChEBI ID | 193796 |
|---|
| PubChem Compound ID | 20844 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|