| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:04:40 UTC |
|---|
| Update Date | 2016-11-09 01:22:21 UTC |
|---|
| Accession Number | CHEM040919 |
|---|
| Identification |
|---|
| Common Name | 1,2,3,4-Tetrahydro-beta-carboline |
|---|
| Class | Small Molecule |
|---|
| Description | Tetrahydro-b-carbolines (THbCs)are potential neuroactive alkaloids found in chocolate and cocoa. The formation of 1,2,3,4-tetrahydro-/3-carbolines (THBCs), via the Pictet-Spengler condensation of tryptamines with formaldehyde, has been demonstrated repeatedly in incubations of various mammalian tissues containing added indolethylamine substrate and the methyl donors .5-methyltetrahydrofolate (5-MTHF) or S-adenosylmethionine(SAM). It is concluded that the formation of these THBCs is an artifact produced by the enzymatic liberation of formaldehyde from the methyl donors and the subsequent non-enzymatic condensation of this formaldehyde with the indole substrates. The formation of THBCs in vivo has thus remained a point of contention. (PMID: 7213417). 1,2,3,4-Tetrahydro-beta-carboline is a biomarker for the consumption of beer |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
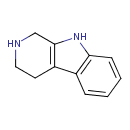
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,2,3,4-Tetrahydro-b-carboline | Generator | | 1,2,3,4-Tetrahydro-β-carboline | Generator | | 1,2,3,4-Tetrahydronorharmane | HMDB | | THBC | HMDB | | Tryptoline hydrochloride | MeSH, HMDB | | THbetaC | MeSH, HMDB | | Tetrahydronorharmane | MeSH, HMDB | | 9H-1,2,3,4-tetrahydropyrido(3,4-b)Indole | MeSH, HMDB | | Noreleagnine | MeSH, HMDB | | tetrahydro-beta-Carboline | MeSH, HMDB | | Tryptoline monohydrochloride | MeSH, HMDB | | Triptoline | MeSH, HMDB | | 1,2,3,4-Tetrahydro-beta-carboline | MeSH | | Tryptoline | MeSH |
|
|---|
| Chemical Formula | C11H12N2 |
|---|
| Average Molecular Mass | 172.226 g/mol |
|---|
| Monoisotopic Mass | 172.100 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 1H,2H,3H,4H,9H-pyrido[3,4-b]indole |
|---|
| Traditional Name | tryptoline |
|---|
| SMILES | C1CC2=C(CN1)NC1=C2C=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C11H12N2/c1-2-4-10-8(3-1)9-5-6-12-7-11(9)13-10/h1-4,12-13H,5-7H2 |
|---|
| InChI Key | CFTOTSJVQRFXOF-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as beta carbolines. Beta carbolines are compounds containing a 9H-pyrido[3,4-b]indole moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Indoles and derivatives |
|---|
| Sub Class | Pyridoindoles |
|---|
| Direct Parent | Beta carbolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Beta-carboline
- 3-alkylindole
- Indole
- Aralkylamine
- Benzenoid
- Heteroaromatic compound
- Pyrrole
- Azacycle
- Secondary amine
- Secondary aliphatic amine
- Organopnictogen compound
- Organonitrogen compound
- Amine
- Organic nitrogen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0596-0900000000-0c12ea5ede1ecce0b8a0 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-0006-3900000000-59256f2013af0388b609 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0006-0900000000-298d086f2db7bfb96c7f | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0006-3900000000-59256f2013af0388b609 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-0006-0900000000-0e95cd13061c8268d13d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0900000000-d1cf77f85d424207ea20 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-006x-0900000000-fa3e868e9ca6a59d5b91 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0560-0900000000-76a12e37b5faa4cbb856 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0900000000-2305b865b13eae850bbb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0900000000-d58681edbdcc31aced92 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0596-0900000000-3e4b9943ce69d390f2aa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0900000000-ff5f70dda818b9673030 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0900000000-1b2e7c8ce9fa45740ef0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00bc-2900000000-f5466bd5eff0535cb5b2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0900000000-84df739a23ca1993145c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0900000000-84df739a23ca1993145c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-0900000000-234e77ff29e2c2061761 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0012488 |
|---|
| FooDB ID | FDB029095 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00052418 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 96979 |
|---|
| ChEBI ID | 100417 |
|---|
| PubChem Compound ID | 107838 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Barker SA, Harrison RE, Monti JA, Brown GB, Christian ST: Identification and quantification of 1,2,3,4-tetrahydro-beta-carboline, 2-methyl-1,2,3,4-tetrahydro-beta-carboline, and 6-methoxy-1,2,3,4-tetrahydro-beta-carboline as in vivo constituents of rat brain and adrenal gland. Biochem Pharmacol. 1981 Jan 1;30(1):9-17. |
|
|---|