| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 01:04:17 UTC |
|---|
| Update Date | 2016-11-09 01:22:21 UTC |
|---|
| Accession Number | CHEM040904 |
|---|
| Identification |
|---|
| Common Name | (-)-Salsoline |
|---|
| Class | Small Molecule |
|---|
| Description | (-)-Salsoline is a compound that crystallizes from alcohol solution, melts at 221 oC, soluble in hot alcohol and chloroform; used in medicine as an antihypertensive agent. Salsoline as well as salsolinol were found in male alcoholic inpatients's urine and lumbar cerebrospinal fluid when patients were still intoxicated after a heavy alcohol debauch and after they had been inpatients and off alcohol for one week.There was a wide interindividual variation and no statistical significant difference in the levels between the first and second sampling in CSF or urine.[PMID: 6935920]. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
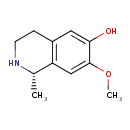
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+)-Salsoline | MeSH | | (S)-Salsoline | MeSH | | 1(R),2(N)-Dimethyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline | MeSH | | 7-O-Methylsalsolinol | MeSH | | D-Salosine | MeSH | | N-Methyl-(R)-salsolinol | MeSH | | Methylsalsolinol | MeSH | | Salsoline | MeSH | | Salsoline (-)-form | MeSH | | Salsoline hydrochloride | MeSH | | Salsoline hydrochloride, (R)-isomer | MeSH | | Salsoline hydrochloride, (S)-isomer | MeSH | | Salsoline hydrochloride, hydrate (4:4:1) | MeSH | | (1S)-7-Methoxy-1-methyl-1,2,3,4-tetrahydroisoquinolin-6-ol | HMDB |
|
|---|
| Chemical Formula | C11H15NO2 |
|---|
| Average Molecular Mass | 193.242 g/mol |
|---|
| Monoisotopic Mass | 193.110 g/mol |
|---|
| CAS Registry Number | 89-31-6 |
|---|
| IUPAC Name | (1S)-7-methoxy-1-methyl-1,2,3,4-tetrahydroisoquinolin-6-ol |
|---|
| Traditional Name | salsoline |
|---|
| SMILES | COC1=CC2=C(CCN[C@H]2C)C=C1O |
|---|
| InChI Identifier | InChI=1S/C11H15NO2/c1-7-9-6-11(14-2)10(13)5-8(9)3-4-12-7/h5-7,12-13H,3-4H2,1-2H3/t7-/m0/s1 |
|---|
| InChI Key | YTPRLBGPGZHUPD-ZETCQYMHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tetrahydroisoquinolines. These are tetrahydrogenated isoquinoline derivatives. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrahydroisoquinolines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Tetrahydroisoquinolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tetrahydroisoquinoline
- Anisole
- Alkyl aryl ether
- 1-hydroxy-2-unsubstituted benzenoid
- Aralkylamine
- Benzenoid
- Secondary aliphatic amine
- Ether
- Secondary amine
- Azacycle
- Organooxygen compound
- Organonitrogen compound
- Amine
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fb9-0900000000-46a08ac1000ef9e8d2e6 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0fk9-7790000000-6cf66e441b1984402c51 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0900000000-60174f1cf4cc1f4b4834 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0900000000-503253f6315fa936ceab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0072-2900000000-65a6dbd945d9089bf6ab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-00e30f946db869ed3635 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0900000000-919b64fcfb6b74a436dc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01ea-4900000000-b5c88563d80dfdd1ba10 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-5326ed117e0a68853a6c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-1900000000-4e91134b5e3127909ce9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-2900000000-0a98cc8b818bd78f9081 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0900000000-093c204a145d72b06e8c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002f-0900000000-5fcd8088e3a540d3cdaf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ae9-3900000000-ba42a8e3e1235ca4e8c2 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0012469 |
|---|
| FooDB ID | FDB029079 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00001914 C00027481 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 390808 |
|---|
| ChEBI ID | 761542 |
|---|
| PubChem Compound ID | 442356 |
|---|
| Kegg Compound ID | C09640 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|