| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 00:33:29 UTC |
|---|
| Update Date | 2016-11-09 01:22:19 UTC |
|---|
| Accession Number | CHEM040781 |
|---|
| Identification |
|---|
| Common Name | 3-O-Sulfogalactosylceramide (d18:1/24:1(15Z)) |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
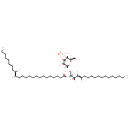
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (15E)-N-[(2S,3R,4E)-1-{[(2R,5S,6R)-3,5-dihydroxy-6-(hydroxymethyl)-4-(sulfooxy)oxan-2-yl]oxy}-3-hydroxyoctadec-4-en-2-yl]tetracos-15-enimidate | Generator | | (15E)-N-[(2S,3R,4E)-1-{[(2R,5S,6R)-3,5-dihydroxy-6-(hydroxymethyl)-4-(sulphooxy)oxan-2-yl]oxy}-3-hydroxyoctadec-4-en-2-yl]tetracos-15-enimidate | Generator | | (15E)-N-[(2S,3R,4E)-1-{[(2R,5S,6R)-3,5-dihydroxy-6-(hydroxymethyl)-4-(sulphooxy)oxan-2-yl]oxy}-3-hydroxyoctadec-4-en-2-yl]tetracos-15-enimidic acid | Generator |
|
|---|
| Chemical Formula | C48H91NO11S |
|---|
| Average Molecular Mass | 890.310 g/mol |
|---|
| Monoisotopic Mass | 889.631 g/mol |
|---|
| CAS Registry Number | 151057-28-2 |
|---|
| IUPAC Name | (15E)-N-[(2S,3R,4E)-1-{[(2R,5S,6R)-3,5-dihydroxy-6-(hydroxymethyl)-4-(sulfooxy)oxan-2-yl]oxy}-3-hydroxyoctadec-4-en-2-yl]tetracos-15-enimidic acid |
|---|
| Traditional Name | (15E)-N-[(2S,3R,4E)-1-{[(2R,5S,6R)-3,5-dihydroxy-6-(hydroxymethyl)-4-(sulfooxy)oxan-2-yl]oxy}-3-hydroxyoctadec-4-en-2-yl]tetracos-15-enimidic acid |
|---|
| SMILES | [H]\C(CCCCCCCC)=C(\[H])CCCCCCCCCCCCCC(O)=N[C@@]([H])(CO[C@]1([H])O[C@]([H])(CO)[C@]([H])(O)C([H])(OS(O)(=O)=O)C1([H])O)[C@]([H])(O)C(\[H])=C(/[H])CCCCCCCCCCCCC |
|---|
| InChI Identifier | InChI=1S/C48H91NO11S/c1-3-5-7-9-11-13-15-17-18-19-20-21-22-23-24-26-28-30-32-34-36-38-44(52)49-41(42(51)37-35-33-31-29-27-25-16-14-12-10-8-6-4-2)40-58-48-46(54)47(60-61(55,56)57)45(53)43(39-50)59-48/h17-18,35,37,41-43,45-48,50-51,53-54H,3-16,19-34,36,38-40H2,1-2H3,(H,49,52)(H,55,56,57)/b18-17+,37-35+/t41-,42+,43+,45-,46?,47?,48+/m0/s1 |
|---|
| InChI Key | ZZQWQNAZXFNSEP-CRPGRBEASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as glycosphingolipids. These are sphingolipids containing a saccharide moiety glycosidically attached to the sphingoid base. Although saccharide moieties are mostly O-glycosidically linked to the ceramide moiety, other sphingolipids with glycosidic bonds of other types (e.g. S-,C-, or N-type) has been reported. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Sphingolipids |
|---|
| Sub Class | Glycosphingolipids |
|---|
| Direct Parent | Glycosphingolipids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Glycosphingolipid
- Fatty acyl glycoside of mono- or disaccharide
- Fatty acyl glycoside
- Hexose monosaccharide
- Alkyl glycoside
- O-glycosyl compound
- Glycosyl compound
- Fatty acyl
- Sulfuric acid ester
- Alkyl sulfate
- Sulfate-ester
- Sulfuric acid monoester
- Oxane
- Monosaccharide
- Organic sulfuric acid or derivatives
- Secondary alcohol
- Oxacycle
- Organoheterocyclic compound
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboximidic acid derivative
- Carboximidic acid
- Acetal
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dl-0010043090-b7053392356ac277ed61 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-008a-0033049240-c7318fdd3d58a456d1b8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-7298033880-3153459e59d1be6cb92f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0079-0021015090-929c15c134e4b8edfd4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052f-6164019580-ed89b4edbd0f1a824393 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9353101000-bf4aa0a6e659b100f815 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|