| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 00:33:13 UTC |
|---|
| Update Date | 2016-11-09 01:22:19 UTC |
|---|
| Accession Number | CHEM040779 |
|---|
| Identification |
|---|
| Common Name | 3-O-Sulfogalactosylceramide (d18:1/22:0) |
|---|
| Class | Small Molecule |
|---|
| Description | 3-O-Sulfogalactosylceramide is an acidic, sulfated glycosphingolipid, often known as sulfatide. This lipid occurs in membranes of various cell types, but is found in particularly high concentrations in myelin where it constitutes 3-4% of total membrane lipids. This lipid is synthesized primarily in the oligodendrocytes in the central nervous system. Accumulation of this lipid in the lysosomes is a characteristic of metachromatic leukodystrophy, a lysosomal storage disease caused by the deficiency of arylsulfatase A. Alterations in sulfatide metabolism, trafficking, and homeostasis are present in the earliest clinically recognizable stages of Alzheimer's disease.Cerebrosides are glycosphingolipids. There are four types of glycosphingolipids, the cerebrosides, sulfatides, globosides and gangliosides. Cerebrosides have a single sugar group linked to ceramide. The most common are galactocerebrosides (containing galactose), the least common are glucocerebrosides (containing glucose). Galactocerebrosides are found predominantly in neuronal cell membranes. In contrast glucocerebrosides are not normally found in membranes. Instead, they are typically intermediates in the synthesis or degradation of more complex glycosphingolipids. Galactocerebrosides are synthesized from ceramide and UDP-galactose. Excess lysosomal accumulation of glucocerebrosides is found in Gaucher disease. Sulfatides are glycosphingolipids. There are four types of glycosphingolipids, the cerebrosides, sulfatides, globosides and gangliosides. Sulfatides are the sulfuric acid esters of galactocerebrosides. They are synthesized from galactocerebrosides and activated sulfate, 3'-phosphoadenosine 5'-phosphosulfate (PAPS). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
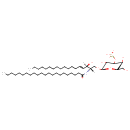
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-O-Sulphogalactosylceramide (D18:1/22:0) | Generator | | 3'-O-Sulphogalactosylceramide | HMDB | | 3-O-SulfO-beta-D-galactosylceramide | HMDB | | 3-O-SulfO-beta-delta-galactosylceramide | HMDB | | 3-O-Sulfogalactosylceramide | HMDB | | Cerebroside 3-sulfate | HMDB | | Cerebroside 3-sulphate | HMDB | | Galactosylceramide-sulfate | HMDB | | Galactosylceramide-sulphate | HMDB | | Galactosylceramidesulfate | HMDB | | Galactosylceramidesulphate | HMDB | | N-[(1S,2R,3E)-2-Hydroxy-1-[[(3-O-sulfO-b-D-galactopyranosyl)oxy]methyl]-3-heptadecen-1-yl]-docosanamide | HMDB | | N-[(1S,2R,3E)-2-Hydroxy-1-[[(3-O-sulfO-beta-delta-galactopyranosyl)oxy]methyl]-3-heptadecen-1-yl]-docosanamide | HMDB | | Sulfatide | HMDB | | Sulfatide (D18:1/22:0) | HMDB | | [R-[R*,s*-(e)]]-N-[2-hydroxy-1-[[(3-O-sulfO-b-D-galactopyranosyl)oxy]methyl]-3-heptadecenyl]-docosanamide | HMDB | | [R-[R*,s*-(e)]]-N-[2-hydroxy-1-[[(3-O-sulfO-beta-delta-galactopyranosyl)oxy]methyl]-3-heptadecenyl]-docosanamide | HMDB | | (3'-SulphO)galbeta-cer(D18:1/22:0) | Generator |
|
|---|
| Chemical Formula | C46H89NO11S |
|---|
| Average Molecular Mass | 864.264 g/mol |
|---|
| Monoisotopic Mass | 863.616 g/mol |
|---|
| CAS Registry Number | 265096-83-1 |
|---|
| IUPAC Name | [(2R,5S,6R)-2-{[(2S,3R,4E)-2-docosanamido-3-hydroxyoctadec-4-en-1-yl]oxy}-3,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxidanesulfonic acid |
|---|
| Traditional Name | C22 sulfatide |
|---|
| SMILES | CCCCCCCCCCCCCCCCCCCCCC(=O)N[C@@]([H])(CO[C@@H]1O[C@H](CO)[C@H](O)C(OS(=O)(O)=O)C1O)[C@@](O)([H])\C=C\CCCCCCCCCCCCC |
|---|
| InChI Identifier | InChI=1S/C46H89NO11S/c1-3-5-7-9-11-13-15-17-18-19-20-21-22-24-26-28-30-32-34-36-42(50)47-39(40(49)35-33-31-29-27-25-23-16-14-12-10-8-6-4-2)38-56-46-44(52)45(58-59(53,54)55)43(51)41(37-48)57-46/h33,35,39-41,43-46,48-49,51-52H,3-32,34,36-38H2,1-2H3,(H,47,50)(H,53,54,55)/b35-33+/t39-,40+,41+,43-,44?,45?,46+/m0/s1 |
|---|
| InChI Key | UAKYQMHTPLVMJD-GJOJTGTQSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sulfatides. These are an hydrogen sulfate esters of glycosphingolipids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Sphingolipids |
|---|
| Sub Class | Glycosphingolipids |
|---|
| Direct Parent | Sulfatides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sulfoglycosphingolipid
- Glycosyl-n-acylsphingosine
- Fatty acyl glycoside
- Fatty acyl glycoside of mono- or disaccharide
- Alkyl glycoside
- Hexose monosaccharide
- Glycosyl compound
- O-glycosyl compound
- Fatty amide
- Monosaccharide
- N-acyl-amine
- Oxane
- Fatty acyl
- Sulfuric acid monoester
- Sulfate-ester
- Alkyl sulfate
- Sulfuric acid ester
- Organic sulfuric acid or derivatives
- Carboxamide group
- Secondary carboxylic acid amide
- Secondary alcohol
- Organoheterocyclic compound
- Carboxylic acid derivative
- Acetal
- Oxacycle
- Organic oxide
- Organic oxygen compound
- Primary alcohol
- Organonitrogen compound
- Organooxygen compound
- Carbonyl group
- Organic nitrogen compound
- Hydrocarbon derivative
- Alcohol
- Organopnictogen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000000090-ea81e5ad2dc3824cc930 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-1161011290-74e761e439460cf44662 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-053s-9232001000-4fd13f0bccabe6eee8b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-2000013090-537be9077f9aad482c4e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-8020039230-de5449e2307880e38037 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-053v-9140020000-292816f881b4e89b65e1 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0012316 |
|---|
| FooDB ID | FDB028938 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | GALACTOSYLCERAMIDE-SULFATE |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 24823252 |
|---|
| ChEBI ID | 176327 |
|---|
| PubChem Compound ID | 24779582 |
|---|
| Kegg Compound ID | C06125 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|