| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 00:31:23 UTC |
|---|
| Update Date | 2016-11-09 01:22:19 UTC |
|---|
| Accession Number | CHEM040755 |
|---|
| Identification |
|---|
| Common Name | Prephenate |
|---|
| Class | Small Molecule |
|---|
| Description | An oxo dicarboxylic acid that consists of 4-hydroxycyclohexa-2,5-diene-1-carboxylic acid substituted by a 2-carboxy-2-oxoethyl group at position 1. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
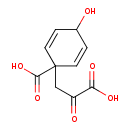
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Carboxy-4-hydroxy-2,5-cyclohexadiene-1-pyruvic acid | ChEBI | | 1-Carboxy-4-hydroxy-alpha-oxo-2,5-cyclohexadiene-1-propanoic acid | ChEBI | | 1-Carboxy-4-hydroxy-2,5-cyclohexadiene-1-pyruvate | Generator | | 1-Carboxy-4-hydroxy-a-oxo-2,5-cyclohexadiene-1-propanoate | Generator | | 1-Carboxy-4-hydroxy-a-oxo-2,5-cyclohexadiene-1-propanoic acid | Generator | | 1-Carboxy-4-hydroxy-alpha-oxo-2,5-cyclohexadiene-1-propanoate | Generator | | 1-Carboxy-4-hydroxy-α-oxo-2,5-cyclohexadiene-1-propanoate | Generator | | 1-Carboxy-4-hydroxy-α-oxo-2,5-cyclohexadiene-1-propanoic acid | Generator | | Prephenic acid | Generator | | PRE | HMDB |
|
|---|
| Chemical Formula | C10H10O6 |
|---|
| Average Molecular Mass | 226.183 g/mol |
|---|
| Monoisotopic Mass | 226.048 g/mol |
|---|
| CAS Registry Number | 126-49-8 |
|---|
| IUPAC Name | 1-(2-carboxy-2-oxoethyl)-4-hydroxycyclohexa-2,5-diene-1-carboxylic acid |
|---|
| Traditional Name | prephenic acid |
|---|
| SMILES | OC1C=CC(CC(=O)C(O)=O)(C=C1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C10H10O6/c11-6-1-3-10(4-2-6,9(15)16)5-7(12)8(13)14/h1-4,6,11H,5H2,(H,13,14)(H,15,16) |
|---|
| InChI Key | FPWMCUPFBRFMLH-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as gamma-keto acids and derivatives. These are organic compounds containing an aldehyde substituted with a keto group on the C4 carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Keto acids and derivatives |
|---|
| Sub Class | Gamma-keto acids and derivatives |
|---|
| Direct Parent | Gamma-keto acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Gamma-keto acid
- Dicarboxylic acid or derivatives
- Alpha-keto acid
- Alpha-hydroxy ketone
- Secondary alcohol
- Ketone
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 MEOX; 3 TMS) | splash10-004u-5930000000-ab2b31a4c4dfd40ceddb | Spectrum | | GC-MS | GC-MS Spectrum - GC-MS (1 MEOX; 3 TMS) | splash10-004u-9810000000-ac9f3a0c71b3c5665bf8 | Spectrum | | GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-014u-8910000000-3415a97033f86fa8c8a8 | Spectrum | | GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-004u-5930000000-ab2b31a4c4dfd40ceddb | Spectrum | | GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-004u-9810000000-ac9f3a0c71b3c5665bf8 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9610000000-b3ced3fa2b10936d8535 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-004l-8009100000-38ca188548d74699664b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0960000000-977e79544dc4467ac727 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0bu9-1910000000-ad1d0e019512e236b48e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-3900000000-328dfece2609b4273be8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-1790000000-740f63025e2d1901bfcc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-06v0-1920000000-23a6bf28fe9915f6fca3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000f-8900000000-a949dc8f9457a7974670 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0012283 |
|---|
| FooDB ID | FDB028914 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00019632 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | PREPHENATE |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Prephenic_acid |
|---|
| Chemspider ID | 1001 |
|---|
| ChEBI ID | 16666 |
|---|
| PubChem Compound ID | 1028 |
|---|
| Kegg Compound ID | C00254 |
|---|
| YMDB ID | YMDB00324 |
|---|
| ECMDB ID | ECMDB12283 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|