| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 00:30:31 UTC |
|---|
| Update Date | 2016-11-09 01:22:19 UTC |
|---|
| Accession Number | CHEM040744 |
|---|
| Identification |
|---|
| Common Name | Molybdopterin-AMP |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
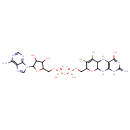
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Adenylated molybdopterin | HMDB |
|
|---|
| Chemical Formula | C20H26N10O12P2S2 |
|---|
| Average Molecular Mass | 724.558 g/mol |
|---|
| Monoisotopic Mass | 724.065 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | ({2-amino-4-oxo-6,7-disulfanyl-3H,4H,5H,5aH,8H,9aH,10H-pyrano[3,2-g]pteridin-8-yl}methoxy)[({[5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy]phosphinic acid |
|---|
| Traditional Name | {2-amino-4-oxo-6,7-disulfanyl-3H,5H,5aH,8H,9aH,10H-pyrano[3,2-g]pteridin-8-yl}methoxy({[5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy(hydroxy)phosphoryl}oxy)phosphinic acid |
|---|
| SMILES | NC1=NC2=C(NC3C(N2)OC(COP(O)(=O)OP(O)(=O)OCC2OC(C(O)C2O)N2C=NC4=C2N=CN=C4N)C(S)=C3S)C(=O)N1 |
|---|
| InChI Identifier | InChI=1S/C20H26N10O12P2S2/c21-14-8-16(24-3-23-14)30(4-25-8)19-11(32)10(31)5(41-19)1-38-43(34,35)42-44(36,37)39-2-6-12(45)13(46)7-18(40-6)27-15-9(26-7)17(33)29-20(22)28-15/h3-7,10-11,18-19,26,31-32,45-46H,1-2H2,(H,34,35)(H,36,37)(H2,21,23,24)(H4,22,27,28,29,33) |
|---|
| InChI Key | XJXFAXLUOKQPAQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as molybdopterin dinucleotides. These are a dinucleotide that is made up of a molybdopterin and a purine or pyrimidine base linked to each other through a phosphate chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Molybdopterin dinucleotides |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Molybdopterin dinucleotides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Molybdopterin dinucleotide
- Purine ribonucleoside diphosphate
- Molybdopterin
- Purine ribonucleoside monophosphate
- Pentose phosphate
- Pentose-5-phosphate
- Pyranopterin
- Pterin
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Monosaccharide phosphate
- Organic pyrophosphate
- Pentose monosaccharide
- Pteridine
- Imidazopyrimidine
- Purine
- Secondary aliphatic/aromatic amine
- Pyrimidone
- Aminopyrimidine
- Monoalkyl phosphate
- Monosaccharide
- N-substituted imidazole
- Pyrimidine
- Pyran
- Organic phosphoric acid derivative
- Alkyl phosphate
- Imidolactam
- Phosphoric acid ester
- Tetrahydrofuran
- Imidazole
- Azole
- Heteroaromatic compound
- Vinylogous amide
- 1,2-diol
- Secondary alcohol
- Azacycle
- Secondary amine
- Thioenol
- Oxacycle
- Alkylthiol
- Organoheterocyclic compound
- Amine
- Organopnictogen compound
- Alcohol
- Organic oxygen compound
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Organosulfur compound
- Organic oxide
- Hydrocarbon derivative
- Primary amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004l-1723920100-bb1613d2f84a938b7a7e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0931101300-8d586fe7752001dd24cb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0911000000-b051063e6b7a0a63f1f6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-1910000000-34bc9592e93856ccd5c2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-08n9-1790232100-43f0c1f38049a8fdaf61 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0910600000-682c0ffebbb9af7a0134 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a7i-3900000000-6aece1e85f04e32c2771 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0001000900-ffa1a59ff1824e74917a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002b-0393011600-d5404022df3e8fd231bf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-0923000000-b412c4768c1614066341 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0000002900-a1df204dd3a6582fbd67 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00gr-9502308500-32959dd120e465001372 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-4905805200-c9d6b2442ad99b0bcf05 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0012262 |
|---|
| FooDB ID | FDB028901 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35032425 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 25245677 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | ECMDB21307 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|