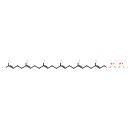| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 00:24:29 UTC |
|---|
| Update Date | 2016-11-09 01:22:18 UTC |
|---|
| Accession Number | CHEM040701 |
|---|
| Identification |
|---|
| Common Name | all-trans-Hexaprenyl diphosphate |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2E,6E,10E,14E,18E)-3,7,11,15,19,23-Hexamethyltetracosa-2,6,10,14,18,22-hexaen-1-yl trihydrogen diphosphate | ChEBI | | (2E,6E,10E,14E,18E)-3,7,11,15,19,23-Hexamethyltetracosa-2,6,10,14,18,22-hexaen-1-yl trihydrogen diphosphoric acid | Generator | | all-trans-Hexaprenyl diphosphoric acid | Generator | | (E)-Hexaprenyl diphosphate | HMDB |
|
|---|
| Chemical Formula | C30H52O7P2 |
|---|
| Average Molecular Mass | 586.677 g/mol |
|---|
| Monoisotopic Mass | 586.319 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | [({[(2E,6E,10E,14E,18E)-3,7,11,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaen-1-yl]oxy}(hydroxy)phosphoryl)oxy]phosphonic acid |
|---|
| Traditional Name | {[(2E,6E,10E,14E,18E)-3,7,11,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaen-1-yl]oxy(hydroxy)phosphoryl}oxyphosphonic acid |
|---|
| SMILES | CC(C)=CCC\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC\C(C)=C\COP(O)(=O)OP(O)(O)=O |
|---|
| InChI Identifier | InChI=1S/C30H52O7P2/c1-25(2)13-8-14-26(3)15-9-16-27(4)17-10-18-28(5)19-11-20-29(6)21-12-22-30(7)23-24-36-39(34,35)37-38(31,32)33/h13,15,17,19,21,23H,8-12,14,16,18,20,22,24H2,1-7H3,(H,34,35)(H2,31,32,33)/b26-15+,27-17+,28-19+,29-21+,30-23+ |
|---|
| InChI Key | NGFSMHKFTZROKJ-MMSZMYIBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as bactoprenol diphosphates. These are polyprenyl compounds consisting of a diphosphate group substituted by a bactoprenyl moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Polyprenols |
|---|
| Direct Parent | Bactoprenol diphosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Bactoprenol diphosphate
- Polyprenyl diphosphate
- Polyprenyl monophosphate
- Sesterterpenoid
- Organic pyrophosphate
- Isoprenoid phosphate
- Monoalkyl phosphate
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00tb-7466390000-1a091a15f69aa600fd33 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0202690000-ca7be5f91d120b34e02f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-2312910000-a18bd263c2252df7e12b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a59-4439820000-433602f028fdf8312339 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0400090000-eba550c0908e0bdcc04f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056r-7900010000-903a330facd80aef97a7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-5db7557b14be93fbdd9b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0100290000-dc5d848846327789fd85 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0aos-0004930000-e9910289e743569a483c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-020a-1925210000-a29abfabad842bde7acf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000090000-8f206fdd3583884650db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-1300190000-fbac398a5a0b0394af5d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-056r-9400000000-c2d5c8e79c928bb87206 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0012188 |
|---|
| FooDB ID | FDB028840 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | ALL-TRANS-HEXAPRENYL-DIPHOSPHATE |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4444089 |
|---|
| ChEBI ID | 17528 |
|---|
| PubChem Compound ID | 5280413 |
|---|
| Kegg Compound ID | C01230 |
|---|
| YMDB ID | YMDB00633 |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|