| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-27 00:20:25 UTC |
|---|
| Update Date | 2016-11-09 01:22:18 UTC |
|---|
| Accession Number | CHEM040668 |
|---|
| Identification |
|---|
| Common Name | 2-Hexaprenyl-6-methoxy-1,4-benzoquinol |
|---|
| Class | Small Molecule |
|---|
| Description | A polyprenylhydroquinone in which the polyprenyl substituent is hexaprenyl at C-2; an additional methoxy group is also present at C-6. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
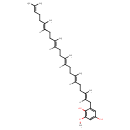
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Hexaprenyl-6-methoxy-1,4-benzoquinol | ChEBI | | 2-Methoxy-6-all-trans-hexaprenyl-1,4-benzoquinol | ChEBI | | 3-Hexaprenyl-5-methoxy-1,4-benzoquinol | ChEBI | | 5-Methoxy-3-hexaprenylhydroquinone | ChEBI | | 5-Methoxy-3-hexaprenylquinol | ChEBI | | 6-Methoxy-2-hexaprenylhydroquinone | ChEBI | | 6-Methoxy-2-hexaprenylquinol | ChEBI |
|
|---|
| Chemical Formula | C37H56O3 |
|---|
| Average Molecular Mass | 548.852 g/mol |
|---|
| Monoisotopic Mass | 548.423 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 2-[(2E,6E,10E,14E,18E)-3,7,11,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaen-1-yl]-6-methoxybenzene-1,4-diol |
|---|
| Traditional Name | 5-methoxy-3-hexaprenylquinol |
|---|
| SMILES | [H]\C(CC\C(C)=C(/[H])CC\C(C)=C(/[H])CC\C(C)=C(/[H])CC\C(C)=C(/[H])CC1=CC(O)=CC(OC)=C1O)=C(\C)CCC=C(C)C |
|---|
| InChI Identifier | InChI=1S/C37H56O3/c1-28(2)14-9-15-29(3)16-10-17-30(4)18-11-19-31(5)20-12-21-32(6)22-13-23-33(7)24-25-34-26-35(38)27-36(40-8)37(34)39/h14,16,18,20,22,24,26-27,38-39H,9-13,15,17,19,21,23,25H2,1-8H3/b29-16+,30-18+,31-20+,32-22+,33-24+ |
|---|
| InChI Key | ZAGWHOPYPMUKOK-FRICUITQSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 2-polyprenyl-6-methoxyphenols. 2-polyprenyl-6-methoxyphenols are compounds containing a polyisoprene chain attached at the 2-position of a 6-methoxyphenol group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Polyprenylphenols |
|---|
| Direct Parent | 2-polyprenyl-6-methoxyphenols |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2-polyprenyl-6-methoxyphenol
- Polyprenylbenzoquinol
- Sesterterpenoid
- Prenylbenzoquinol
- Methoxyphenol
- Anisole
- Hydroquinone
- Phenoxy compound
- Phenol ether
- Methoxybenzene
- Phenol
- Alkyl aryl ether
- 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- Benzenoid
- Ether
- Organooxygen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | - 2-methoxy-6-all-trans-polyprenylhydroquinone (CHEBI:61472 )
|
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0223090000-fb0d8223831f2542b877 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f95-2689430000-628d7903ae3713c64175 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a5a-1389410000-e539e8934cdd393b9c15 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0000090000-6852c53f45c311ec26b5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0000190000-c7da31de373ac1902a8c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fzj-2200980000-647b0a7fca1df1faf7c6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0010940000-f7d8b2679612010370d7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0g4i-0433900000-af7b81f8b391a8ac1689 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zmj-0759700000-696c4e0c81c877bd5113 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0000090000-8df433f72927aa4cdcd9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0uds-0900030000-7f30b5a3a9845e11ef13 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0faa-1925110000-e4c4926f1ffe3cf10526 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0304075 |
|---|
| FooDB ID | FDB030344 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 26332237 |
|---|
| ChEBI ID | 61472 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | ECMDB23144 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|