| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 20:36:09 UTC |
|---|
| Update Date | 2016-11-09 01:22:15 UTC |
|---|
| Accession Number | CHEM040404 |
|---|
| Identification |
|---|
| Common Name | beta-1,4-D-Mannosylchitobiosyldiphosphodolichol |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
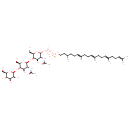
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| b-1,4-D-Mannosylchitobiosyldiphosphodolichol | Generator | | Β-1,4-D-mannosylchitobiosyldiphosphodolichol | Generator | | b-(1->4)-D-mannosylchitobiosyldiphosphodolichol | HMDB | | beta-(1->4)-D-mannosylchitobiosyldiphosphodolichol | HMDB | | beta-(1->4)-delta-mannosylchitobiosyldiphosphodolichol | HMDB | | beta-1,4-delta-Mannosylchitobiosyldiphosphodolichol | HMDB | | beta-D-Mannosyldiacetylchitobiosyldiphosphodolichol | HMDB | | beta-delta-Mannosyldiacetylchitobiosyldiphosphodolichol | HMDB | | Man-beta1->4glcnac-beta1->4glcnac-PP-dol | HMDB | | N-[(2S,3R,4R,5S,6R)-4-Hydroxy-2-{[(2R,3S,4R,5R,6R)-4-hydroxy-6-{[hydroxy({[hydroxy({[(6E,10E,14E)-3,7,11,15,19-pentamethylicosa-6,10,14,18-tetraen-1-yl]oxy})phosphoryl]oxy})phosphoryl]oxy}-5-[(1-hydroxyethylidene)amino]-2-(hydroxymethyl)oxan-3-yl]oxy}-6-(hydroxymethyl)-5-{[(2S,3S,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxan-3-yl]ethanimidate | HMDB |
|
|---|
| Chemical Formula | C47H82N2O22P2 |
|---|
| Average Molecular Mass | 1089.102 g/mol |
|---|
| Monoisotopic Mass | 1088.483 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | {[(2R,3R,4R,5S,6R)-3-acetamido-5-{[(2S,3R,4R,5S,6R)-3-acetamido-4-hydroxy-6-(hydroxymethyl)-5-{[(2S,3S,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxan-2-yl]oxy}-4-hydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}({[hydroxy({[(6E,10E,14E)-3,7,11,15,19-pentamethylicosa-6,10,14,18-tetraen-1-yl]oxy})phosphoryl]oxy})phosphinic acid |
|---|
| Traditional Name | [(2R,3R,4R,5S,6R)-3-acetamido-5-{[(2S,3R,4R,5S,6R)-3-acetamido-4-hydroxy-6-(hydroxymethyl)-5-{[(2S,3S,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxan-2-yl]oxy}-4-hydroxy-6-(hydroxymethyl)oxan-2-yl]oxy({hydroxy[(6E,10E,14E)-3,7,11,15,19-pentamethylicosa-6,10,14,18-tetraen-1-yl]oxyphosphoryl}oxy)phosphinic acid |
|---|
| SMILES | OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](NC(=O)C)[C@@H](O[C@@H]2CO)O[C@H]2[C@H](O)[C@@H](NC(=O)C)[C@H](O[C@@H]2CO)OP(=O)(O)OP(=O)(O)OCCC(C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CCC=C(C)C)[C@@H](O)[C@@H](O)[C@@H]1O |
|---|
| InChI Identifier | InChI=1S/C47H82N2O22P2/c1-26(2)13-9-14-27(3)15-10-16-28(4)17-11-18-29(5)19-12-20-30(6)21-22-64-72(60,61)71-73(62,63)70-46-37(49-32(8)54)40(57)43(35(25-52)67-46)68-45-36(48-31(7)53)39(56)44(34(24-51)66-45)69-47-42(59)41(58)38(55)33(23-50)65-47/h13,15,17,19,30,33-47,50-52,55-59H,9-12,14,16,18,20-25H2,1-8H3,(H,48,53)(H,49,54)(H,60,61)(H,62,63)/b27-15+,28-17+,29-19+/t30?,33-,34-,35-,36-,37-,38-,39-,40-,41+,42+,43-,44-,45+,46-,47+/m1/s1 |
|---|
| InChI Key | CMBCFQGXXHOGEH-LSMBNOQWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as acyclic diterpenoids. These are diterpenoids (compounds made of four consecutive isoprene units) that do not contain a cycle. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Diterpenoids |
|---|
| Direct Parent | Acyclic diterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acyclic diterpenoid
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- N-acyl-l-alpha-amino acid
- Cysteine or derivatives
- Alpha-amino acid or derivatives
- N-acyl-amine
- Secondary carboxylic acid amide
- Carboxamide group
- Alkylthiol
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic oxygen compound
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Organosulfur compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0597-9004010114-de697cc038745af3f74c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052f-2119330204-60687570e93ae5acb5f8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-2329310014-fe696fe548cc70e9db62 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-3482950000-9ac2d17f15601ded1f63 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fvi-9512160512-3dc048458007d2f79fbc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pdl-3901330000-bbec6d21c700735a20f6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-9000000000-63fde6f7ba9f34f93cb1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-02di-9202000302-bac1ce5762d5fa0fd1e7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0zfr-8950150412-b1c3bbd81d8735270d87 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-003i-9001000012-38cce817270368bac505 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001j-9210000010-44302c6b7e71301324f0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000x-9842001001-a8d084685b1f1ffa51b0 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0011673 |
|---|
| FooDB ID | FDB028362 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | D-GALACTOSYL-14-D-GALACTOSYL-14-D- |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 26333131 |
|---|
| ChEBI ID | 18396 |
|---|
| PubChem Compound ID | 45259170 |
|---|
| Kegg Compound ID | C05860 |
|---|
| YMDB ID | YMDB00189 |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|