| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 20:34:36 UTC |
|---|
| Update Date | 2016-11-09 01:22:14 UTC |
|---|
| Accession Number | CHEM040361 |
|---|
| Identification |
|---|
| Common Name | Lactosylceramide (d18:1/24:0) |
|---|
| Class | Small Molecule |
|---|
| Description | Lactosylceramide (d18:1/24:0) is a lactosylceramide or LacCer. Lactosylceramides are the most important and abundant of the diosylceramides. Lactosylceramides (LacCer) were originally called 'cytolipin H'. It is found in small amounts only in most animal tissues, but it has a number of significant biological functions and it is of great importance as the biosynthetic precursor of most of the neutral oligoglycosylceramides, sulfatides and gangliosides. In animal tissues, biosynthesis of lactosylceramide involves addition of the second monosaccharides unit (galactose) as its nucleotide derivative to monoglucosylceramide, catalysed by a specific beta-1,4-galactosyltransferase on the lumenal side of the Golgi apparatus. The glucosylceramide precursor must first cross from the cytosolic side of the membrane, possibly via the action of a flippase. The lactosylceramide produced can be further glycosylated or transferred to the plasma membrane. Lactosylceramide may assist in stabilizing the plasma membrane and activating receptor molecules in the special micro-domains or rafts, as with the cerebrosides. It may also have its own specialized function in the immunological system in that it is known to bind to specific bacteria. In addition, it is believed that a number of pro-inflammatory factors activate lactosylceramide synthase to generate lactosylceramide, which in turn activates "oxygen-sensitive" signalling pathways that affect such cellular processes as proliferation, adhesion, migration and angiogenesis. Dysfunctions in these pathways can affect several diseases of the cardiovascular system, cancer and inflammatory states, so lactosylceramide metabolism is a potential target for new therapeutic treatments. beta-D-Galactosyl-1,4-beta-D-glucosylceramide is the second to last step in the synthesis of N-Acylsphingosine and is converted
from Glucosylceramide via the enzyme beta-1,4-galactosyltransferase 6(EC:2.4.1.-). It can be converted to Glucosylceramide via the enzyme beta-galactosidase (EC:3.2.1.23). [HMDB] |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
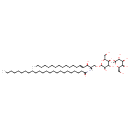
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| LacCer(D18:1/24:0) | ChEBI | | N-(Tetracosanoyl)-1-b-lactosyl-sphing-4-enine | ChEBI | | b-D-Galactosyl-(1->4)-b-D-glucosyl-(11)-N-tetracosanoylsphingosine | Generator, HMDB | | β-D-Galactosyl-(1->4)-β-D-glucosyl-(11)-N-tetracosanoylsphingosine | Generator, HMDB | | Lactosylceramide | MetBuilder | | N-(Tetracosanoyl)-1-β-lactosyl-sphing-4-enine | MetBuilder | | Lactosylceramide(d18:1/24:0) | MetBuilder | | N-(Tetracosanoyl)-1-β-lactosyl-sphingosine | MetBuilder | | N-(Tetracosanoyl)-1-β-lactosyl-D-erythro-sphingosine | MetBuilder | | N-(Tetracosanoyl)-1-β-lactosyl-4-sphingenine | MetBuilder | | N-(Tetracosanoyl)-1-β-lactosyl-D-sphingosine | MetBuilder | | N-(Tetracosanoyl)-1-β-lactosyl-sphingenine | MetBuilder | | N-(Tetracosanoyl)-1-β-lactosyl-erythro-4-sphingenine | MetBuilder | | LacCer d18:1/24:0 | HMDB | | Lactosylceramide (d18:1,C24:0) | HMDB | | Lactosylceramide (d18:1/24:0) | HMDB | | N-Lignoceroylsphingosyl lactoside | HMDB |
|
|---|
| Chemical Formula | C54H103NO13 |
|---|
| Average Molecular Mass | 974.395 g/mol |
|---|
| Monoisotopic Mass | 973.743 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | N-[(2S,3R,4E)-1-{[(2R,3R,4R,5S,6R)-3,4-dihydroxy-6-(hydroxymethyl)-5-{[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxan-2-yl]oxy}-3-hydroxyoctadec-4-en-2-yl]tetracosanamide |
|---|
| Traditional Name | N-[(2S,3R,4E)-1-{[(2R,3R,4R,5S,6R)-3,4-dihydroxy-6-(hydroxymethyl)-5-{[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxan-2-yl]oxy}-3-hydroxyoctadec-4-en-2-yl]tetracosanamide |
|---|
| SMILES | [H][C@@](CO[C@@H]1O[C@H](CO)[C@@H](O[C@@H]2O[C@H](CO)[C@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O)(NC(=O)CCCCCCCCCCCCCCCCCCCCCCC)[C@]([H])(O)\C=C\CCCCCCCCCCCCC |
|---|
| InChI Identifier | InChI=1S/C54H103NO13/c1-3-5-7-9-11-13-15-17-18-19-20-21-22-23-24-26-28-30-32-34-36-38-46(59)55-42(43(58)37-35-33-31-29-27-25-16-14-12-10-8-6-4-2)41-65-53-51(64)49(62)52(45(40-57)67-53)68-54-50(63)48(61)47(60)44(39-56)66-54/h35,37,42-45,47-54,56-58,60-64H,3-34,36,38-41H2,1-2H3,(H,55,59)/b37-35+/t42-,43+,44+,45+,47-,48-,49+,50+,51+,52+,53+,54-/m0/s1 |
|---|
| InChI Key | KDEYEEYMIPNKIJ-OGIIFMLESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as glycosyl-n-acylsphingosines. Glycosyl-N-acylsphingosines are compounds containing a sphingosine linked to a simple glucosyl moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Sphingolipids |
|---|
| Sub Class | Glycosphingolipids |
|---|
| Direct Parent | Glycosyl-N-acylsphingosines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Glycosyl-n-acylsphingosine
- Fatty acyl glycoside
- Fatty acyl glycoside of mono- or disaccharide
- Alkyl glycoside
- Disaccharide
- Glycosyl compound
- O-glycosyl compound
- Fatty amide
- N-acyl-amine
- Oxane
- Fatty acyl
- Secondary carboxylic acid amide
- Secondary alcohol
- Carboxamide group
- Organoheterocyclic compound
- Polyol
- Oxacycle
- Carboxylic acid derivative
- Acetal
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Alcohol
- Organonitrogen compound
- Carbonyl group
- Organooxygen compound
- Primary alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | - beta-D-galactosyl-(1->4)-beta-D-glucosyl-(1<->1)-N-acylsphingosine (CHEBI:84764 )
|
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0btc-0102005529-5a7c2cd0cb37ce86e633 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01po-0213209832-0136b0b482b6376feeba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03l3-1719714563-e0bb814c60dfdb529aeb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0fk9-0312004239-690f4c6a9ff6c6dfaba4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0itc-4705026934-50f237a6f4960e03675c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01t9-4902102020-f37f0974711d18799ab4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03e9-2400009254-418c48c406bc350be9d3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01qc-3900005301-dcc34f2668b2a28bd4c5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02tc-4910005100-89706dc05f3fb692a3e1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0101000109-63682271662de10e23c5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-06rx-7593002325-d7f40628549239745531 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-5329513000-dc8535504008afee0c70 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0011595 |
|---|
| FooDB ID | FDB028308 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 24765753 |
|---|
| ChEBI ID | 84764 |
|---|
| PubChem Compound ID | 53481001 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|