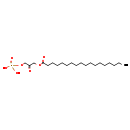
DHAP(18:0) (CHEM039968)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2016-05-26 19:25:25 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2016-11-09 01:22:10 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | CHEM039968 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | DHAP(18:0) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | DHAP(18:0) is the octadecanoyl derivative of Dihydroxyacetone phosphate. It is also known as an alkyl-DHAP. This compound is formed by octadecanoic acid reacting with DHAP. Alkyl-DHAPs are intermediates in the synthesis of ether phospholipids. The initial steps of ether phospholipid biosynthesis take place in peroxisomes. Alkyl-dihydroxyacetonephosphate synthase is the peroxisomal enzyme that actually introduces the ether linkage. Levels of Alkyl-DHAP have been found to be strongly reduced in human fibroblasts derived from Zellweger syndrome and rhizomelic chondrodysplasia punctata patients. Four other enzymes are known to be involved in the metabolism of acyl-DHAP and alkyl-DHAP. These include: acyl-DHAP/alkyl-DHAP oxidoreductase, DHAP acyltransferase, alkyl-DHAP phosphohydrolase, and a dinitrofluorobenzene-insensitive acyl-DHAP acylhydrolase. Dihydroxyacetone phosphate (DHAP) is a biochemical compound primarily involved in the glycolysis metabolic pathway. DHAP is also the product of the dehydrogenation of L-glycerol-3-phosphate which is part of the entry of glycerol (sourced from triglycerides) into the glycolytic pathway. Conversely, reduction of glycolysis-derived DHAP to L-glycerol-3-phosphate provides adipose cells with the activated glycerol backbone they require to synthesize new triglycerides. Both reactions are catalyzed by the enzyme glycerol 3-phosphate dehydrogenase with NAD+/NADH as cofactor. DHAP may be referred to as glycerone phosphate in older texts. 1-Octadecyl-glycerone-3-phosphate is an intermediate in Ether lipid metabolism. DHAP(18:0) or 1-Octadecanoyl-glycerone-3-phosphate is the precursor to 1-Octadecyl-glycerone-3-phosphate DHAP(18:0e) which is generated via alkylglycerone phosphate synthase (EC: 2.5.1.26). Ether lipids are lipids in which one or more of the carbon atoms on glycerol is bonded to an alkyl chain via an ether linkage, as opposed to the usual ester linkage. Ether lipids are called plasmalogens (1-O-1'-alkenyl-2-acylglycerophospholipids) if these are glycerol-containing phospholipids with an unsaturated O-(1-alkenyl) (vinyl ether) group at the first position on the glycerol chain. Plasmalogens as well as some 1-O-alkyl lipids are ubiquitous and sometimes major parts of the cell membranes in mammals and anaerobic bacteria. In archaea, ether lipids are the major polar lipids in the cell envelope and their abundance is one of the major characteristics that separate this group of prokaryotes from the bacteria. In these cells, diphytanylglycerolipids or bipolar macrocyclic tetraethers can form covalently linked 'bilayers'. [HMDB] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Contaminant Sources |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Contaminant Type | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C21H41O7P | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 436.520 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 436.259 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | [3-(octadecanoyloxy)-2-oxopropoxy]phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | stearoylglycerone phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | CCCCCCCCCCCCCCCCCC(=O)OCC(=O)COP(O)(O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C21H41O7P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-21(23)27-18-20(22)19-28-29(24,25)26/h2-19H2,1H3,(H2,24,25,26) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | GTPATKZCXDKGQS-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as o-acylglycerone-phosphates. These are glycerone-3-phosphates carrying an acyl substituent at the 1-position. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic oxygen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Organooxygen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Carbonyl compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | O-acylglycerone-phosphates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | HMDB0011133 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FooDB ID | FDB027912 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phenol Explorer ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KNApSAcK ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BiGG ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| METLIN ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemspider ID | 389126 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | 36476 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 440127 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kegg Compound ID | C03805 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| YMDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ECMDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||