| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 19:22:16 UTC |
|---|
| Update Date | 2016-11-09 01:22:10 UTC |
|---|
| Accession Number | CHEM039934 |
|---|
| Identification |
|---|
| Common Name | (R)-3-Hydroxydecanoic acid |
|---|
| Class | Small Molecule |
|---|
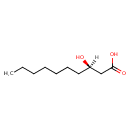
| Description | (R)-3-Hydroxydecanoic acid, also known as (r)-(r)-(r)-3-hydroxydecanoic acid or (r)-3-hydroxydecanoic acid, belongs to the class of organic compounds known as medium-chain hydroxy acids and derivatives. These are hydroxy acids with a 6 to 12 carbon atoms long side chain (R)-3-Hydroxydecanoic acid is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral (R)-3-Hydroxydecanoic acid exists in all eukaryotes, ranging from yeast to humans (R)-3-Hydroxydecanoic acid participates in a number of enzymatic reactions, within cattle. In particular, (R)-3-Hydroxydecanoic acid can be biosynthesized from 3-oxodecanoic acid through the action of the enzyme fatty acid synthase. Beta ketoacyl synthase domain. In addition, (R)-3-Hydroxydecanoic acid can be converted into trans-dec-2-enoic acid through the action of the enzyme fatty acid synthase. dyhydrase domain. In cattle, (R)-(r)-(r)-3-hydroxydecanoic acid is involved in the metabolic pathway called fatty acid biosynthesis pathway. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (R)-3-Hydroxydecanoate | Generator | | 3-HDA | HMDB | | 3-Hydroxydecanoic acid | HMDB | | beta-Hydroxydecanoic acid | HMDB | | Myrmicacin, (+-)-isomer | HMDB | | Myrmicacin monosodium (+-)-isomer | HMDB | | 3-Hydroxy-decanoic acid | HMDB | | Myrmicacin, (R)-isomer | HMDB | | Myrmicacin | HMDB |
|
|---|
| Chemical Formula | C10H20O3 |
|---|
| Average Molecular Mass | 188.264 g/mol |
|---|
| Monoisotopic Mass | 188.141 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (3R)-3-hydroxydecanoic acid |
|---|
| Traditional Name | (R)-3-hydroxydecanoic acid |
|---|
| SMILES | [H][C@@](O)(CCCCCCC)CC(O)=O |
|---|
| InChI Identifier | InChI=1S/C10H20O3/c1-2-3-4-5-6-7-9(11)8-10(12)13/h9,11H,2-8H2,1H3,(H,12,13)/t9-/m1/s1 |
|---|
| InChI Key | FYSSBMZUBSBFJL-SECBINFHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as medium-chain hydroxy acids and derivatives. These are hydroxy acids with a 6 to 12 carbon atoms long side chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Hydroxy acids and derivatives |
|---|
| Sub Class | Medium-chain hydroxy acids and derivatives |
|---|
| Direct Parent | Medium-chain hydroxy acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Medium-chain hydroxy acid
- Medium-chain fatty acid
- Beta-hydroxy acid
- Hydroxy fatty acid
- Fatty acyl
- Fatty acid
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic oxide
- Alcohol
- Carbonyl group
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-007c-9200000000-f9795d95896f66d90766 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-010c-9041000000-b9b51f3624f22e4b6c9d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dr-0900000000-1d9eae6ad3d5e431996b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fmr-3900000000-bbd0b333dda24219033a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9200000000-7cc23eacacf566b10ff0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-1900000000-46d09f9537cea04f6e13 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-054x-3900000000-76f4dd284af0c499a0a8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a6u-9400000000-84da3410c532bb2e2273 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-1900000000-2019592125745914c6e0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a6r-9800000000-b6a04af2348e32c41166 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9000000000-8f7fd17dc3ad7497c377 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-009i-9800000000-2661614f9f11754f0d3a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4l-9000000000-b7cbaa25fb90f53def32 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9000000000-6609ad3e1fed5bc9c4ca | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0010725 |
|---|
| FooDB ID | FDB027873 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4472223 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5312798 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | YMDB16207 |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|