| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 18:00:42 UTC |
|---|
| Update Date | 2016-11-09 01:22:06 UTC |
|---|
| Accession Number | CHEM039584 |
|---|
| Identification |
|---|
| Common Name | Bilirubin glucuronide |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
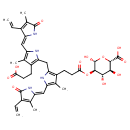
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,17-Diethenyl-1,10-19,22,23,24-hexahydro-3,7,13,18-tetramethyl-1,19-dioxo-21H-biline-8,12-dipropanoate | HMDB | | 2,17-Diethenyl-1,10-19,22,23,24-hexahydro-3,7,13,18-tetramethyl-1,19-dioxo-21H-biline-8,12-dipropanoic acid | HMDB | | 2,17-Diethenyl-1,10-19,22,23,24-hexahydro-3,7,13,18-tetramethyl-1,19-dioxo-21H-biline-8,12-dipropanoic acid mono-beta-D-glucopyranuronosyl ester | HMDB | | 2,17-Diethenyl-1,10-19,22,23,24-hexahydro-3,7,13,18-tetramethyl-1,19-dioxo-21H-biline-8,12-dipropanoic acid mono-beta-delta-glucopyranuronosyl ester | HMDB | | Bilirubin glucuronate | HMDB | | Bilirubin glucuronic acid conjugates | HMDB | | Bilirubin monoglucuronide | HMDB | | Glucuronic acid conjugates OF bilirubin | HMDB | | mono-beta-delta-Glucopyranuronosyl ester 2,17-diethenyl-1,10-19,22,23,24-hexahydro-3,7,13,18-tetramethyl-1,19-dioxo-21H-biline-8,12-dipropanoate | HMDB | | mono-beta-delta-Glucopyranuronosyl ester 2,17-diethenyl-1,10-19,22,23,24-hexahydro-3,7,13,18-tetramethyl-1,19-dioxo-21H-biline-8,12-dipropanoic acid | HMDB | | (2S,3S,4S,5R,6R)-5-{[3-(2-{[3-(2-carboxyethyl)-5-{[(2Z)-3-ethenyl-5-hydroxy-4-methyl-2H-pyrrol-2-ylidene]methyl}-4-methyl-1H-pyrrol-2-yl]methyl}-5-{[(2Z)-4-ethenyl-5-hydroxy-3-methyl-2H-pyrrol-2-ylidene]methyl}-4-methyl-1H-pyrrol-3-yl)propanoyl]oxy}-3,4,6-trihydroxyoxane-2-carboxylate | HMDB | | Glucuronate conjugates OF bilirubin | HMDB |
|
|---|
| Chemical Formula | C39H44N4O12 |
|---|
| Average Molecular Mass | 760.786 g/mol |
|---|
| Monoisotopic Mass | 760.296 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (2S,3S,4S,5R,6R)-5-{[3-(2-{[3-(2-carboxyethyl)-5-{[(2Z)-3-ethenyl-4-methyl-5-oxo-2,5-dihydro-1H-pyrrol-2-ylidene]methyl}-4-methyl-1H-pyrrol-2-yl]methyl}-5-{[(2Z)-4-ethenyl-3-methyl-5-oxo-2,5-dihydro-1H-pyrrol-2-ylidene]methyl}-4-methyl-1H-pyrrol-3-yl)propanoyl]oxy}-3,4,6-trihydroxyoxane-2-carboxylic acid |
|---|
| Traditional Name | (2S,3S,4S,5R,6R)-5-{[3-(2-{[3-(2-carboxyethyl)-5-{[(2Z)-3-ethenyl-4-methyl-5-oxo-1H-pyrrol-2-ylidene]methyl}-4-methyl-1H-pyrrol-2-yl]methyl}-5-{[(2Z)-4-ethenyl-3-methyl-5-oxo-1H-pyrrol-2-ylidene]methyl}-4-methyl-1H-pyrrol-3-yl)propanoyl]oxy}-3,4,6-trihydroxyoxane-2-carboxylic acid |
|---|
| SMILES | CC1=C(C=C)\C(NC1=O)=C\C1=C(C)C(CCC(O)=O)=C(CC2=C(CCC(=O)O[C@H]3[C@H](O)O[C@@H]([C@@H](O)[C@@H]3O)C(O)=O)C(C)=C(N2)\C=C2/NC(=O)C(C=C)=C2C)N1 |
|---|
| InChI Identifier | InChI=1S/C39H44N4O12/c1-7-20-19(6)36(49)43-27(20)14-25-17(4)22(9-11-30(44)45)28(41-25)15-29-23(18(5)24(40-29)13-26-16(3)21(8-2)37(50)42-26)10-12-31(46)54-35-33(48)32(47)34(38(51)52)55-39(35)53/h7-8,13-14,32-35,39-41,47-48,53H,1-2,9-12,15H2,3-6H3,(H,42,50)(H,43,49)(H,44,45)(H,51,52)/b26-13-,27-14-/t32-,33-,34-,35+,39+/m0/s1 |
|---|
| InChI Key | NCZCFMOVDHRJII-VBORGTJOSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as bilirubins. These are organic compounds containing a dicarboxylic acyclic tetrapyrrole derivative. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Bilirubins |
|---|
| Direct Parent | Bilirubins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Bilirubin skeleton
- Glucuronic acid or derivatives
- Hexose monosaccharide
- Tricarboxylic acid or derivatives
- Beta-hydroxy acid
- Fatty acid ester
- Monosaccharide
- Hydroxy acid
- Oxane
- Fatty acyl
- Substituted pyrrole
- Pyran
- Heteroaromatic compound
- Pyrrole
- Pyrroline
- Secondary carboxylic acid amide
- Hemiacetal
- Lactam
- Carboxylic acid ester
- 1,2-diol
- Carboxamide group
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Oxacycle
- Azacycle
- Polyol
- Alcohol
- Hydrocarbon derivative
- Organic nitrogen compound
- Organic oxide
- Carbonyl group
- Organic oxygen compound
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_7) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_8) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_9) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_5) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_6) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_7) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_8) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_9) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002f-0300030900-0865b86b083bbe063f3b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00os-0900150700-a3d05b0eb0cb1285c343 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004j-3900134200-ca8293fb4addf5c11e9e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-067l-2400044900-10373cf294707957940e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00lu-4700091400-55082bd1b554e6e72fad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05o0-6300090000-9b0c34749943f4dd7f98 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-057i-9100077600-20e650c74f102f68001c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-053l-7300097300-9da993c164d046106b50 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000l-5090671000-d3c50eb55fd5219cae71 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01ox-0010120900-f4caa7fa954314bd2ce3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-02tc-0010332900-c5e0d05638e77b1c92c0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0v0r-0895346200-dcb90c3f6d2d07f63a12 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0010332 |
|---|
| FooDB ID | FDB027484 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | 41733 |
|---|
| BioCyc ID | Beta-D-Glucuronides |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Bilirubin glucuronide |
|---|
| Chemspider ID | 4942827 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 6438344 |
|---|
| Kegg Compound ID | C03033 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|