| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 18:00:35 UTC |
|---|
| Update Date | 2016-11-09 01:22:06 UTC |
|---|
| Accession Number | CHEM039582 |
|---|
| Identification |
|---|
| Common Name | Phenethylamine glucuronide |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
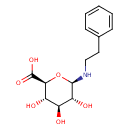
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Phenylethylamine glucuronide | HMDB | | (2S,3S,4S,5R,6R)-3,4,5-Trihydroxy-6-[(2-phenylethyl)amino]oxane-2-carboxylate | HMDB |
|
|---|
| Chemical Formula | C14H19NO6 |
|---|
| Average Molecular Mass | 297.304 g/mol |
|---|
| Monoisotopic Mass | 297.121 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (2S,3S,4S,5R,6R)-3,4,5-trihydroxy-6-[(2-phenylethyl)amino]oxane-2-carboxylic acid |
|---|
| Traditional Name | phenethylamine glucuronide |
|---|
| SMILES | O[C@@H]1[C@@H](O)[C@H](NCCC2=CC=CC=C2)O[C@@H]([C@H]1O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C14H19NO6/c16-9-10(17)12(14(19)20)21-13(11(9)18)15-7-6-8-4-2-1-3-5-8/h1-5,9-13,15-18H,6-7H2,(H,19,20)/t9-,10-,11+,12-,13+/m0/s1 |
|---|
| InChI Key | OYNLGXUYULDVEJ-IEECTRCBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-glucuronides. These are glucuronides in which the aglycone is linked to the carbohydrate unit through a N-glycosidic bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | N-glucuronides |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-glucuronide
- 1-n-glucuronide
- Glycosyl compound
- N-glycosyl compound
- Beta-hydroxy acid
- Monocyclic benzene moiety
- Hydroxy acid
- Monosaccharide
- Oxane
- Pyran
- Benzenoid
- Amino acid
- Amino acid or derivatives
- Secondary alcohol
- Hemiaminal
- Polyol
- Oxacycle
- Organoheterocyclic compound
- Secondary amine
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Carboxylic acid
- Secondary aliphatic amine
- Organonitrogen compound
- Organopnictogen compound
- Organic oxide
- Carbonyl group
- Alcohol
- Amine
- Hydrocarbon derivative
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a6r-9440000000-53feb5385bf85eb9990d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-00di-3122490000-d086a68a8f89f70181d7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-008a-0390000000-c36c12c05b9bd705cc9c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ab9-1920000000-b61f7b736af2bb008eac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-4900000000-749286a7ce9ce348b6d2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01ot-0890000000-3f93998103c3e2900a5b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00el-2920000000-98736ca1b61facb0441b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05fs-7900000000-ec5e4aa12a33ac96e57d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0032-0090000000-69ce0cdd12ed53cd0c9b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05bg-6490000000-80037d60a671c4948c93 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9610000000-1ada3099cf31a1833c57 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-059t-0960000000-76b0d6f4811f27fda574 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a59-1970000000-f7c4c059de3e872d1c62 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-054o-9600000000-4f079ef34b5091a692f8 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0010323 |
|---|
| FooDB ID | FDB027475 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Beta-D-Glucuronides |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 166036 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 191195 |
|---|
| Kegg Compound ID | C03033 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|