| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 17:12:05 UTC |
|---|
| Update Date | 2016-11-09 01:22:05 UTC |
|---|
| Accession Number | CHEM039479 |
|---|
| Identification |
|---|
| Common Name | SM(d18:0/16:0) |
|---|
| Class | Small Molecule |
|---|
| Description | SM(d18:0/16:0) (d18:0/16:0) or SM(d18:0/16:0)is a type of sphingolipid found in animal cell membranes, especially in the membranous myelin sheath which surrounds some nerve cell axons. It usually consists of phosphorylcholine and ceramide. SM(18:0/16:0) consists of stearic acid attached to the C1 position and palmitic acid attached to the C2 position. In humans, SM(d18:0/16:0) is the only membrane phospholipid not derived from glycerol. Like all sphingolipids, SPH has a ceramide core (sphingosine bonded to a fatty acid via an amide linkage). In addition it contains one polar head group, which is either phosphocholine or phosphoethanolamine. The plasma membrane of cells is highly enriched in SM(d18:0/16:0) and is considered largely to be found in the exoplasmic leaflet of the cell membrane. However, there is some evidence that there may also be a SM(d18:0/16:0) pool in the inner leaflet of the membrane. Moreover, neutral sphingomyelinase-2 - an enzyme that breaks down SM(d18:0/16:0) into ceramide has been found to localise exclusively to the inner leaflet further suggesting that there may be SM(d18:0/16:0) present there. SM(d18:0/16:0) can accumulate in a rare hereditary disease called Niemann-Pick Disease, types A and B. Niemann-Pick disease is a genetically-inherited disease caused by a deficiency in the enzyme Sphingomyelinase, which causes the accumulation of SM(d18:0/16:0) in spleen, liver, lungs, bone marrow, and the brain, causing irreversible neurological damage. SMs play a role in signal transduction.
Sphingomyelins are synthesized by the transfer of phosphorylcholine from phosphatidylcholine to a ceramide in a reaction catalyzed by SM(d18:0/16:0) synthase. |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
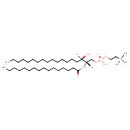
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| C16 Sphingomyelin | HMDB | | N-(Hexadecanoyl)-sphing-4-enine-1-phosphocholine | HMDB | | Sphingomyelin | HMDB | | Sphingomyelin (D18:0/16:0) | HMDB | | N-(Hexadecanoyl)-1-phosphocholine-sphinganine | MetBuilder | | Sphingomyelin(D18:0/16:0) | MetBuilder | | N-(Hexadecanoyl)-1-phosphocholine-dihydrosphingosine | MetBuilder | | N-(Hexadecanoyl)-1-phosphocholine-D-erythro-sphinganine | MetBuilder |
|
|---|
| Chemical Formula | C39H82N2O6P |
|---|
| Average Molecular Mass | 706.052 g/mol |
|---|
| Monoisotopic Mass | 705.591 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | {[(2S,3R)-2-hexadecanamido-3-hydroxyoctadecyl]oxy}[2-(trimethylazaniumyl)ethoxy]phosphinic acid |
|---|
| Traditional Name | [(2S,3R)-2-hexadecanamido-3-hydroxyoctadecyl]oxy(2-(trimethylammonio)ethoxy)phosphinic acid |
|---|
| SMILES | [H][C@@](O)(CCCCCCCCCCCCCCC)[C@]([H])(COP(O)(=O)OCC[N+](C)(C)C)NC(=O)CCCCCCCCCCCCCCC |
|---|
| InChI Identifier | InChI=1S/C39H81N2O6P/c1-6-8-10-12-14-16-18-20-22-24-26-28-30-32-38(42)37(36-47-48(44,45)46-35-34-41(3,4)5)40-39(43)33-31-29-27-25-23-21-19-17-15-13-11-9-7-2/h37-38,42H,6-36H2,1-5H3,(H-,40,43,44,45)/p+1/t37-,38+/m0/s1 |
|---|
| InChI Key | QHZIGNLCLJPLCU-QPPIDDCLSA-O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phosphosphingolipids. These are sphingolipids with a structure based on a sphingoid base that is attached to a phosphate head group. They differ from phosphonospingolipids which have a phosphonate head group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Sphingolipids |
|---|
| Sub Class | Phosphosphingolipids |
|---|
| Direct Parent | Phosphosphingolipids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sphingoid-1-phosphate or derivatives
- Phosphocholine
- Phosphoethanolamine
- Dialkyl phosphate
- Fatty amide
- N-acyl-amine
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Fatty acyl
- Alkyl phosphate
- Tetraalkylammonium salt
- Quaternary ammonium salt
- Secondary carboxylic acid amide
- Secondary alcohol
- Carboxamide group
- Carboxylic acid derivative
- Organic salt
- Amine
- Alcohol
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic cation
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | Not Available |
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0010168 |
|---|
| FooDB ID | FDB027351 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4446704 |
|---|
| ChEBI ID | 17636 |
|---|
| PubChem Compound ID | 5283591 |
|---|
| Kegg Compound ID | C00550 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|