| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 12:24:09 UTC |
|---|
| Update Date | 2016-11-09 01:21:49 UTC |
|---|
| Accession Number | CHEM038141 |
|---|
| Identification |
|---|
| Common Name | PE(14:0/14:0) |
|---|
| Class | Small Molecule |
|---|
| Description | PE(14:0/14:0), also known as pe(14:0/14:0) or PE(28:0), belongs to the class of organic compounds known as phosphatidylethanolamines. These are glycerophosphoetahnolamines in which two fatty acids are bonded to the glycerol moiety through ester linkages. Thus, PE(14:0/14:0) is considered to be a glycerophosphoethanolamine lipid molecule. PE(14:0/14:0) is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. PE(14:0/14:0) exists in all living species, ranging from bacteria to humans. PE(14:0/14:0) participates in a number of enzymatic reactions, within cattle. In particular, Cytidine monophosphate and PE(14:0/14:0) can be biosynthesized from CDP-ethanolamine and DG(14:0/14:0/0:0); which is mediated by the enzyme choline/ethanolaminephosphotransferase. Furthermore, PE(14:0/14:0) can be biosynthesized from PS(14:0/14:0) through its interaction with the enzyme phosphatidylserine decarboxylase. Furthermore, PE(14:0/14:0) can be biosynthesized from PS(14:0/14:0); which is catalyzed by the enzyme phosphatidylserine decarboxylase. Finally, Cytidine monophosphate and PE(14:0/14:0) can be biosynthesized from CDP-ethanolamine and DG(14:0/14:0/0:0); which is mediated by the enzyme choline/ethanolaminephosphotransferase. In cattle, PE(14:0/14:0) is involved in a couple of metabolic pathways, which include phosphatidylcholine biosynthesis PC(14:0/14:0) pathway and phosphatidylethanolamine biosynthesis pe(14:0/14:0) pathway. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
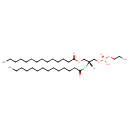
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,2-Dimyristoylphosphatidylethanolamine | HMDB | | DMPE | HMDB | | PE(28:0) | HMDB | | GPEtn(28:0) | HMDB | | GPEtn(14:0/14:0) | HMDB | | Phophatidylethanolamine(28:0) | HMDB | | 1,2-Dimyristoyl-rac-glycero-3-phosphoethanolamine | HMDB | | 1,2-Ditetradecanoyl-rac-glycero-3-phosphoethanolamine | HMDB | | Phophatidylethanolamine(14:0/14:0) | HMDB | | 1,2-Dimyristoyl-sn-glycero-3-phosphoethanolamine | HMDB | | 1,2-Ditetradecanoyl-sn-glycero-3-phosphoethanolamine | HMDB | | Dimyristoyl cephalin | HMDB | | Tetradecanoic acid, (1R)-1-((((2-aminoethoxy)hydroxyphosphinyl)oxy)methyl)-1,2-ethanediyl ester | HMDB | | PE(14:0/14:0) | Lipid Annotator |
|
|---|
| Chemical Formula | C33H66NO8P |
|---|
| Average Molecular Mass | 635.864 g/mol |
|---|
| Monoisotopic Mass | 635.453 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (2-aminoethoxy)[(2R)-2,3-bis(tetradecanoyloxy)propoxy]phosphinic acid |
|---|
| Traditional Name | 2-aminoethoxy((2R)-2,3-bis(tetradecanoyloxy)propoxy)phosphinic acid |
|---|
| SMILES | [H][C@@](COC(=O)CCCCCCCCCCCCC)(COP(O)(=O)OCCN)OC(=O)CCCCCCCCCCCCC |
|---|
| InChI Identifier | InChI=1S/C33H66NO8P/c1-3-5-7-9-11-13-15-17-19-21-23-25-32(35)39-29-31(30-41-43(37,38)40-28-27-34)42-33(36)26-24-22-20-18-16-14-12-10-8-6-4-2/h31H,3-30,34H2,1-2H3,(H,37,38)/t31-/m1/s1 |
|---|
| InChI Key | NEZDNQCXEZDCBI-WJOKGBTCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phosphatidylethanolamines. These are glycerophosphoetahnolamines in which two fatty acids are bonded to the glycerol moiety through ester linkages. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerophospholipids |
|---|
| Sub Class | Glycerophosphoethanolamines |
|---|
| Direct Parent | Phosphatidylethanolamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diacylglycero-3-phosphoethanolamine
- Phosphoethanolamine
- Fatty acid ester
- Dialkyl phosphate
- Dicarboxylic acid or derivatives
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Fatty acyl
- Amino acid or derivatives
- Carboxylic acid ester
- Carboxylic acid derivative
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Amine
- Primary aliphatic amine
- Organic nitrogen compound
- Primary amine
- Carbonyl group
- Organic oxide
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0010009000-6d6f74a433c2e2723cd1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0010009000-6d6f74a433c2e2723cd1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0059-0390306000-9038cf0f0478e5f02ff9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0000109000-470823ccf44c645a8bac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000b-0010905000-0e4cba84714254189647 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-0010902000-a9f87916897ba156fbfe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0010009000-b19e2ffec592b767d0e6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0010009000-b19e2ffec592b767d0e6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0059-0390306000-04e550c53d4828b11284 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0000019000-999c75a9b12dda2125cf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0aor-0000019000-94fd3647bcad36d1803a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-0101019000-1d5f2b3017f1e9f4051c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0000109000-cb4377400ad388b2a614 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000b-0010905000-20841fda513aa1793c46 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-0010902000-6f24410a84ced3193b6d | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0008821 |
|---|
| FooDB ID | FDB026011 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 9852308 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | YMDB14022 |
|---|
| ECMDB ID | ECMDB08821 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|