| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 09:07:32 UTC |
|---|
| Update Date | 2016-11-09 01:21:37 UTC |
|---|
| Accession Number | CHEM037051 |
|---|
| Identification |
|---|
| Common Name | DG(22:5(4Z,7Z,10Z,13Z,16Z)/18:4(6Z,9Z,12Z,15Z)/0:0) |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
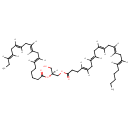
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2S)-3-Hydroxy-2-[(6E,9E,12E,15E)-octadeca-6,9,12,15-tetraenoyloxy]propyl (4E,7E,10E,13E,16E)-docosa-4,7,10,13,16-pentaenoic acid | Generator |
|
|---|
| Chemical Formula | C43H66O5 |
|---|
| Average Molecular Mass | 662.996 g/mol |
|---|
| Monoisotopic Mass | 662.491 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (2S)-3-hydroxy-2-[(6E,9E,12E,15E)-octadeca-6,9,12,15-tetraenoyloxy]propyl (4E,7E,10E,13E,16E)-docosa-4,7,10,13,16-pentaenoate |
|---|
| Traditional Name | (2S)-3-hydroxy-2-[(6E,9E,12E,15E)-octadeca-6,9,12,15-tetraenoyloxy]propyl (4E,7E,10E,13E,16E)-docosa-4,7,10,13,16-pentaenoate |
|---|
| SMILES | [H]\C(CC)=C(\[H])C\C([H])=C(/[H])C\C([H])=C(/[H])C\C([H])=C(/[H])CCCCC(=O)O[C@@]([H])(CO)COC(=O)CC\C([H])=C(/[H])C\C([H])=C(/[H])C\C([H])=C(/[H])C\C([H])=C(/[H])C\C([H])=C(/[H])CCCCC |
|---|
| InChI Identifier | InChI=1S/C43H66O5/c1-3-5-7-9-11-13-15-17-19-20-21-22-24-25-27-29-31-33-35-37-42(45)47-40-41(39-44)48-43(46)38-36-34-32-30-28-26-23-18-16-14-12-10-8-6-4-2/h6,8,11-14,17-19,21-23,25,27-28,30-31,33,41,44H,3-5,7,9-10,15-16,20,24,26,29,32,34-40H2,1-2H3/b8-6+,13-11+,14-12+,19-17+,22-21+,23-18+,27-25+,30-28+,33-31+/t41-/m0/s1 |
|---|
| InChI Key | GZBVGLNVBKBGBN-KZDWJCTQSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,2-diacylglycerols. These are diacylglycerols containing a glycerol acylated at positions 1 and 2. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerolipids |
|---|
| Sub Class | Diradylglycerols |
|---|
| Direct Parent | 1,2-diacylglycerols |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,2-acyl-sn-glycerol
- Fatty acid ester
- Fatty acyl
- Dicarboxylic acid or derivatives
- Carboxylic acid ester
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-08fr-1059104000-9822af8c9fba45b0ef5b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0bu9-2097211000-572933053e0bf2ec8929 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-07ci-1092110000-2fb5beccae8d013b2146 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03fr-0039002000-8e9e42a0270595c0adca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-1029000000-a5a2887a6945e4e07d78 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-5089000000-793e873d615e813ac90e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|