| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 07:21:33 UTC |
|---|
| Update Date | 2016-11-09 01:21:28 UTC |
|---|
| Accession Number | CHEM036296 |
|---|
| Identification |
|---|
| Common Name | 3a,7a,12a-Trihydroxy-5b-24-oxocholestanoyl-CoA |
|---|
| Class | Small Molecule |
|---|
| Description | 3a,7a,12a-Trihydroxy-5b-24-oxocholestanoyl-CoA is an intermediate in bile acid synthesis. Bile acids are steroid acids found predominantly in bile of mammals. The distinction between different bile acids is minute, depends only on presence or absence of hydroxyl groups on positions 3, 7, and 12. Bile acids are physiological detergents that facilitate excretion, absorption, and transport of fats and sterols in the intestine and liver. Bile acids are also steroidal amphipathic molecules derived from the catabolism of cholesterol. They modulate bile flow and lipid secretion, are essential for the absorption of dietary fats and vitamins, and have been implicated in the regulation of all the key enzymes involved in cholesterol homeostasis. Bile acids recirculate through the liver, bile ducts, small intestine and portal vein to form an enterohepatic circuit. They exist as anions at physiological pH and, consequently, require a carrier for transport across the membranes of the enterohepatic tissues. The unique detergent properties of bile acids are essential for the digestion and intestinal absorption of hydrophobic nutrients. Bile acids have potent toxic properties (e.g., membrane disruption) and there are a plethora of mechanisms to limit their accumulation in blood and tissues. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
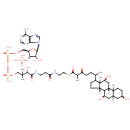
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (24R,25R)-3-alpha,7-alpha,12-alpha,24-Tetrahydroxy-5-beta-cholestan-26-oyl-CoA | HMDB | | (24R,25R)-3-alpha,7-alpha,12-alpha,24-Tetrahydroxy-5-beta-cholestan-26-oyl-coenzyme A | HMDB | | 24-oxo-25(R)-Trihydroxycoprostanoyl-CoA | HMDB | | 24-oxo-25(R)-Trihydroxycoprostanoyl-coenzyme A | HMDB | | 25(R)-24-oxo-3alpha,7alpha,12alpha-Trihydroxycoprostanoyl-CoA | HMDB | | 25(R)-24-oxo-3alpha,7alpha,12alpha-Trihydroxycoprostanoyl-coenzyme A | HMDB | | 25(R)-3alpha,7alpha,12alpha-Trihydroxy-24-oxo-5beta-cholestanoyl-CoA | HMDB | | 25(R)-3alpha,7alpha,12alpha-Trihydroxy-24-oxo-5beta-cholestanoyl-coenzyme A | HMDB | | 25(R)-3alpha,7alpha,12alpha-Trihydroxy-5beta-cholestan-24-on-26-oyl-CoA | HMDB | | 25(R)-3alpha,7alpha,12alpha-Trihydroxy-5beta-cholestan-24-on-26-oyl-coenzyme A | HMDB | | 25(R)-5beta-Cholestane-3alpha,7alpha,12alpha-triol-24-on-26-oyl-CoA | HMDB | | 25(R)-5beta-Cholestane-3alpha,7alpha,12alpha-triol-24-on-26-oyl-coenzyme A | HMDB | | 3-alpha,7-alpha,12-alpha,24-Tetrahydroxy-5-beta-cholestan-26-oyl-CoA | HMDB | | 3-alpha,7-alpha,12-alpha,24-Tetrahydroxy-5-beta-cholestan-26-oyl-coenzyme A | HMDB | | 3-alpha,7-alpha,12-alpha-Trihydroxy-24-oxo-5-beta-cholestanoyl-CoA | HMDB | | 3-alpha,7-alpha,12-alpha-Trihydroxy-24-oxo-5-beta-cholestanoyl-coenzyme A | HMDB | | 3a,7a,12a-Trihydroxy-5b-24-oxocholestanoyl-coenzyme A | HMDB | | 3alpha,7alpha,12alpha-Trihydroxy-24-oxo-5beta-cholestanoyl-CoA | HMDB | | 3alpha,7alpha,12alpha-Trihydroxy-24-oxo-5beta-cholestanoyl-coenzyme A | HMDB | | 3alpha,7alpha,12alpha-Trihydroxy-5beta-24-oxocholestanoyl-CoA | HMDB | | 3alpha,7alpha,12alpha-Trihydroxy-5beta-24-oxocholestanoyl-coenzyme A | HMDB | | R-TrHOCCoA | HMDB | | R-TrHOCcoenzyme A | HMDB |
|
|---|
| Chemical Formula | C48H78N7O21P3S |
|---|
| Average Molecular Mass | 1214.154 g/mol |
|---|
| Monoisotopic Mass | 1213.418 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({[hydroxy({3-hydroxy-2,2-dimethyl-3-[(2-{[2-({2-methyl-3-oxo-6-[(1S,5R,7S,9R,11S,16S)-5,9,16-trihydroxy-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-yl]heptanoyl}sulfanyl)ethyl]carbamoyl}ethyl)carbamoyl]propoxy})phosphoryl]oxy})phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid |
|---|
| Traditional Name | [(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-2-[({hydroxy[hydroxy(3-hydroxy-2,2-dimethyl-3-[(2-{[2-({2-methyl-3-oxo-6-[(1S,5R,7S,9R,11S,16S)-5,9,16-trihydroxy-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-yl]heptanoyl}sulfanyl)ethyl]carbamoyl}ethyl)carbamoyl]propoxy)phosphoryl]oxyphosphoryl}oxy)methyl]oxolan-3-yl]oxyphosphonic acid |
|---|
| SMILES | [H][C@@]12CCC(C(C)CCC(=O)C(C)C(=O)SCCNC(=O)CCNC(=O)C(O)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@H]3O[C@H]([C@H](O)[C@@H]3OP(O)(O)=O)N3C=NC4=C3N=CN=C4N)C1(C)[C@@H](O)C[C@@]1([H])C2[C@H](O)C[C@]2([H])C[C@H](O)CCC12C |
|---|
| InChI Identifier | InChI=1S/C48H78N7O21P3S/c1-24(28-8-9-29-36-30(19-34(59)48(28,29)6)47(5)13-11-27(56)17-26(47)18-32(36)58)7-10-31(57)25(2)45(64)80-16-15-50-35(60)12-14-51-43(63)40(62)46(3,4)21-73-79(70,71)76-78(68,69)72-20-33-39(75-77(65,66)67)38(61)44(74-33)55-23-54-37-41(49)52-22-53-42(37)55/h22-30,32-34,36,38-40,44,56,58-59,61-62H,7-21H2,1-6H3,(H,50,60)(H,51,63)(H,68,69)(H,70,71)(H2,49,52,53)(H2,65,66,67)/t24?,25?,26-,27+,28?,29-,30-,32+,33+,34-,36?,38+,39+,40?,44+,47?,48?/m0/s1 |
|---|
| InChI Key | AWLXQJGPNLCTLM-XFQWJXLTSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 3-oxo-acyl coas. These are organic compounds containing a 3-oxo acylated coenzyme A derivative. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acyl thioesters |
|---|
| Direct Parent | 3-oxo-acyl CoAs |
|---|
| Alternative Parents | |
|---|
| Substituents | - Coenzyme a or derivatives
- Purine ribonucleoside 3',5'-bisphosphate
- Purine ribonucleoside bisphosphate
- Purine ribonucleoside diphosphate
- Steroidal glycoside
- Trihydroxy bile acid, alcohol, or derivatives
- 24-oxosteroid
- Hydroxy bile acid, alcohol, or derivatives
- Cholane-skeleton
- Bile acid, alcohol, or derivatives
- 3-hydroxysteroid
- 12-hydroxysteroid
- Hydroxysteroid
- Oxosteroid
- 3-alpha-hydroxysteroid
- 7-hydroxysteroid
- Steroid
- Ribonucleoside 3'-phosphate
- Pentose phosphate
- Pentose-5-phosphate
- Beta amino acid or derivatives
- Glycosyl compound
- N-glycosyl compound
- Organic pyrophosphate
- 6-aminopurine
- Pentose monosaccharide
- Monosaccharide phosphate
- Purine
- Imidazopyrimidine
- Aminopyrimidine
- Monoalkyl phosphate
- Monosaccharide
- N-acyl-amine
- N-substituted imidazole
- Phosphoric acid ester
- Pyrimidine
- Fatty amide
- 1,3-dicarbonyl compound
- Alkyl phosphate
- Organic phosphoric acid derivative
- Imidolactam
- Azole
- Cyclic alcohol
- Tetrahydrofuran
- Imidazole
- Heteroaromatic compound
- Thiocarboxylic acid ester
- Ketone
- Amino acid or derivatives
- Secondary alcohol
- Carboxamide group
- Secondary carboxylic acid amide
- Carbothioic s-ester
- Polyol
- Organoheterocyclic compound
- Azacycle
- Thiocarboxylic acid or derivatives
- Oxacycle
- Sulfenyl compound
- Carboxylic acid derivative
- Organooxygen compound
- Organic oxygen compound
- Organopnictogen compound
- Carbonyl group
- Organic nitrogen compound
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Primary amine
- Amine
- Organosulfur compound
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-1900021100-1fa27e81efdfbc149a98 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0901132200-5bfed04a7e4f8df63c6c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-1900010100-143578d6a5db8c5e2f2a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00o3-2920642500-18048f2816bacc2e9219 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-003r-3900221010-e4389ba87591b7d47d8b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-057i-5900100000-52d44b7b806816c6bd25 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0290000000-b7a8c3cc183e58c433cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03fu-4770903201-11227f4a396a48a9490f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0170-5122603900-a7af28ea3503a097e921 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03dj-0691000000-af68373f9dfb1b8e7ba0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002r-0951000001-1bcadd0a7e2bbc25d9b2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-0100000900-226355700f943a61d138 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0006891 |
|---|
| FooDB ID | FDB024138 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | 45868 |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 389566 |
|---|
| ChEBI ID | 27379 |
|---|
| PubChem Compound ID | 440690 |
|---|
| Kegg Compound ID | C05467 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|