| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 07:20:22 UTC |
|---|
| Update Date | 2016-11-09 01:21:28 UTC |
|---|
| Accession Number | CHEM036246 |
|---|
| Identification |
|---|
| Common Name | beta-D-Fructose 2-phosphate |
|---|
| Class | Small Molecule |
|---|
| Description | Beta-D-Fructose 2-phosphate, also known as b-D-beta-d-fructose 2-phosphate or 2-O-phosphono-b-D-fructofuranose, belongs to the class of organic compounds known as c-glycosyl compounds. These are glycoside in which a sugar group is bonded through one carbon to another group via a C-glycosidic bond. Beta-D-Fructose 2-phosphate is possibly soluble (in water) and an extremely weak basic (essentially neutral) compound (based on its pKa). Beta-D-Fructose 2-phosphate exists in all living organisms, ranging from bacteria to humans. Beta-D-Fructose 2-phosphate can be converted into D-fructose; which is catalyzed by the enzyme 14 kda phosphohistidine phosphatase. In cattle, Beta-D-fructose 2-phosphate is involved in the metabolic pathway called the fructose and mannose degradation pathway. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
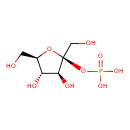
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-O-Phosphono-beta-D-fructofuranose | ChEBI | | 2-Phospho-beta-D-fructofuranose | ChEBI | | beta-D-Fructofuranose 2-phosphate | Kegg | | 2-O-Phosphono-b-D-fructofuranose | Generator | | 2-O-Phosphono-β-D-fructofuranose | Generator | | 2-Phospho-b-D-fructofuranose | Generator | | 2-Phospho-β-D-fructofuranose | Generator | | b-D-Fructofuranose 2-phosphate | Generator | | b-D-Fructofuranose 2-phosphoric acid | Generator | | beta-D-Fructofuranose 2-phosphoric acid | Generator | | Β-D-fructofuranose 2-phosphate | Generator | | Β-D-fructofuranose 2-phosphoric acid | Generator | | b-D-Fructose 2-phosphate | Generator | | b-D-Fructose 2-phosphoric acid | Generator | | beta-D-Fructose 2-phosphoric acid | Generator | | Β-D-fructose 2-phosphate | Generator | | Β-D-fructose 2-phosphoric acid | Generator | | Fructofuranose 2-phosphate | HMDB | | Fructose-2-phosphate | HMDB |
|
|---|
| Chemical Formula | C6H13O9P |
|---|
| Average Molecular Mass | 260.136 g/mol |
|---|
| Monoisotopic Mass | 260.030 g/mol |
|---|
| CAS Registry Number | 19046-69-6 |
|---|
| IUPAC Name | {[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy}phosphonic acid |
|---|
| Traditional Name | fructose-2-phosphate |
|---|
| SMILES | OC[C@H]1O[C@@](CO)(OP(O)(O)=O)[C@@H](O)[C@@H]1O |
|---|
| InChI Identifier | InChI=1S/C6H13O9P/c7-1-3-4(9)5(10)6(2-8,14-3)15-16(11,12)13/h3-5,7-10H,1-2H2,(H2,11,12,13)/t3-,4-,5+,6+/m1/s1 |
|---|
| InChI Key | PMTUDJVZIGZBIX-ZXXMMSQZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as c-glycosyl compounds. These are glycoside in which a sugar group is bonded through one carbon to another group via a C-glycosidic bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | C-glycosyl compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - C-glycosyl compound
- Pentose monosaccharide
- Monoalkyl phosphate
- Monosaccharide
- Organic phosphoric acid derivative
- Alkyl phosphate
- Phosphoric acid ester
- Tetrahydrofuran
- Secondary alcohol
- Organoheterocyclic compound
- Oxacycle
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Primary alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-9110000000-7ca772d08ec8dbad4546 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0gb9-1910100000-560d8b4a6b9fed0746bd | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01ot-9320000000-3273948ba0433a443187 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-9000000000-40d136054d5d0d8b22ff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-009b-9100000000-d6ef0bb32ad7bdbe1069 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4j-9170000000-e3df07bcd35077372295 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9230000000-ebb2e8b2675357e41e81 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-7c59f838aa712f874f34 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-4090000000-93ae68b8c5180dd9006f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9000000000-ef724d5177083af378f0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-9fb2de7e82372b8b1284 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03dj-0950000000-0f2af4d50935260c8249 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03ea-3920000000-8df04f7ec361d949d67b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dj-9600000000-ca86cbbf955b55d79884 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0006800 |
|---|
| FooDB ID | FDB024086 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 167949 |
|---|
| ChEBI ID | 12350 |
|---|
| PubChem Compound ID | 193537 |
|---|
| Kegg Compound ID | C03267 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | ECMDB21663 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|