| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 07:20:00 UTC |
|---|
| Update Date | 2016-11-09 01:21:27 UTC |
|---|
| Accession Number | CHEM036232 |
|---|
| Identification |
|---|
| Common Name | 19-Hydroxytestosterone |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
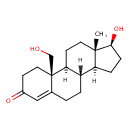
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1H-Indole-5,6-dione | HMDB | | Indole-5,6 quinone | HMDB | | 17beta,19-Dihydroxyandrost-4-en-3-one | HMDB |
|
|---|
| Chemical Formula | C19H28O3 |
|---|
| Average Molecular Mass | 304.424 g/mol |
|---|
| Monoisotopic Mass | 304.204 g/mol |
|---|
| CAS Registry Number | 2126-37-6 |
|---|
| IUPAC Name | (2S,10R,14S,15S)-14-hydroxy-2-(hydroxymethyl)-15-methyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-6-en-5-one |
|---|
| Traditional Name | (2S,10R,14S,15S)-14-hydroxy-2-(hydroxymethyl)-15-methyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-6-en-5-one |
|---|
| SMILES | [H]C12CC[C@H](O)[C@@]1(C)CCC1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12CO |
|---|
| InChI Identifier | InChI=1S/C19H28O3/c1-18-8-7-16-14(15(18)4-5-17(18)22)3-2-12-10-13(21)6-9-19(12,16)11-20/h10,14-17,20,22H,2-9,11H2,1H3/t14-,15?,16?,17-,18-,19+/m0/s1 |
|---|
| InChI Key | YLTCTXBDDHSLCS-HRCCTRRKSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as indoles and derivatives. These are organic compounds containing an indole, which is a bicyclic ring system made up of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Indoles and derivatives |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Indoles and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Indole or derivatives
- Pyrrole
- Vinylogous amide
- Heteroaromatic compound
- Ketone
- Cyclic ketone
- Azacycle
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00g0-0190000000-6e4fc7b300ad16c455cd | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-001i-1203900000-4f90d6dd0388b3a16017 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-0093000000-e6de474519f1a50b8a29 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0670-0090000000-e998b159f6f17e76d6ff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0550-3590000000-c2362be82382a74f0ccb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0059000000-22c9035dbc2909953680 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0zmr-0094000000-a86f4275aef3909e642b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05fs-0090000000-a227eb144908c8f752ba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0019000000-96c876787125565560bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6r-0892000000-64923385891f1c2ddf28 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-0900000000-9b70a6d7d0da7c6a6b3d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0019000000-28d038f60bc762557a4d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0039000000-b8ab70909d16db966311 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ab9-0090000000-21047d70207f0666ac31 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0006779 |
|---|
| FooDB ID | FDB024077 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Indole-5,6-quinone |
|---|
| Chemspider ID | 389600 |
|---|
| ChEBI ID | 27406 |
|---|
| PubChem Compound ID | 440728 |
|---|
| Kegg Compound ID | C05579 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | M2MDB005791 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|