| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 07:19:55 UTC |
|---|
| Update Date | 2016-11-09 01:21:27 UTC |
|---|
| Accession Number | CHEM036229 |
|---|
| Identification |
|---|
| Common Name | 20alpha,22beta-Dihydroxycholesterol |
|---|
| Class | Small Molecule |
|---|
| Description | 20a,22b-Dihydroxycholesterol belongs to the class of organic compounds known as trihydroxy bile acids, alcohols and derivatives. These are prenol lipids structurally characterized by a bile acid or alcohol which bears three hydroxyl groups. 20a,22b-Dihydroxycholesterol is possibly soluble (in water) and an extremely weak basic (essentially neutral) compound (based on its pKa). 20a,22b-Dihydroxycholesterol participates in a number of enzymatic reactions, within cattle. In particular, 20a,22b-Dihydroxycholesterol can be converted into pregnenolone and 4-methylpentanal through the action of the enzyme cholesterol side-chain cleavage enzyme, mitochondrial. In addition, 20a,22b-Dihydroxycholesterol can be biosynthesized from 22b-hydroxycholesterol through the action of the enzyme cholesterol side-chain cleavage enzyme, mitochondrial. In cattle, 20a,22b-dihydroxycholesterol is involved in the metabolic pathway called the steroidogenesis pathway. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
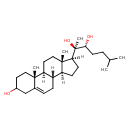
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 20alpha,22beta-Dihydroxycholesterol | HMDB |
|
|---|
| Chemical Formula | C27H46O3 |
|---|
| Average Molecular Mass | 418.652 g/mol |
|---|
| Monoisotopic Mass | 418.345 g/mol |
|---|
| CAS Registry Number | 15234-55-6 |
|---|
| IUPAC Name | (2R,3R)-2-[(1S,2R,10S,11S,14S,15S)-5-hydroxy-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-en-14-yl]-6-methylheptane-2,3-diol |
|---|
| Traditional Name | (2R,3R)-2-[(1S,2R,10S,11S,14S,15S)-5-hydroxy-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-en-14-yl]-6-methylheptane-2,3-diol |
|---|
| SMILES | [H][C@@]12CC[C@]([H])([C@@](C)(O)[C@H](O)CCC(C)C)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2CC(O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C27H46O3/c1-17(2)6-11-24(29)27(5,30)23-10-9-21-20-8-7-18-16-19(28)12-14-25(18,3)22(20)13-15-26(21,23)4/h7,17,19-24,28-30H,6,8-16H2,1-5H3/t19?,20-,21-,22-,23-,24+,25-,26-,27+/m0/s1 |
|---|
| InChI Key | ISBSSBGEYIBVTO-IVMOZYHGSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as trihydroxy bile acids, alcohols and derivatives. These are prenol lipids structurally characterized by a bile acid or alcohol which bears three hydroxyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Trihydroxy bile acids, alcohols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cholesterol
- Cholesterol-skeleton
- Cholestane-skeleton
- Trihydroxy bile acid, alcohol, or derivatives
- 22-hydroxysteroid
- 20-hydroxysteroid
- Hydroxysteroid
- 3-hydroxy-delta-5-steroid
- 3-hydroxysteroid
- Delta-5-steroid
- Cyclic alcohol
- Tertiary alcohol
- Secondary alcohol
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udi-4259400000-35c8f139c691d00dfc8e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-00xr-2111139000-a480b3deb74eef32e383 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uxr-1014900000-d02629f92bcfa0d4d5bd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ab9-8098400000-ec5061b658871254b471 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9065000000-9198f9a283da1d4a6a06 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0003900000-7cd68582455580a1aeeb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01ba-2397500000-8b9915c82bf3ecb52681 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00xr-7289100000-1e37d62442300af9ead1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000900000-f2cb6399034352e924c8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0023900000-85cd6f9f87eb3b745726 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05o1-4139500000-f72eacbae8e6876f643a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0021900000-e2e06cd0437b86637824 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-4596100000-bb184f1626e9252f10ec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-5930000000-979f3659179997a1c795 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0006763 |
|---|
| FooDB ID | FDB024066 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 1294 |
|---|
| PubChem Compound ID | 53477897 |
|---|
| Kegg Compound ID | C05501 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|