| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 07:19:49 UTC |
|---|
| Update Date | 2016-11-09 01:21:27 UTC |
|---|
| Accession Number | CHEM036225 |
|---|
| Identification |
|---|
| Common Name | 11beta,21-Dihydroxy-5beta-pregnane-3,20-dione |
|---|
| Class | Small Molecule |
|---|
| Description | 11b,21-Dihydroxy-5b-pregnane-3,20-dione, also known as 11b,21-dihydroxy-5b-pregnane-3,20-dione, belongs to the class of organic compounds known as 21-hydroxysteroids. These are steroids carrying a hydroxyl group at the 21-position of the steroid backbone. Thus, 11b,21-dihydroxy-5b-pregnane-3,20-dione is considered to be a steroid lipid molecule. 11b,21-Dihydroxy-5b-pregnane-3,20-dione is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. 11b,21-Dihydroxy-5b-pregnane-3,20-dione can be biosynthesized from tetrahydrocorticosterone; which is mediated by the enzyme aldo-keto reductase family 1 member C4. In cattle, 11b,21-dihydroxy-5b-pregnane-3,20-dione is involved in the metabolic pathway called the steroidogenesis pathway. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
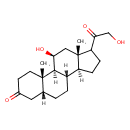
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 11beta,21-Dihydroxy-5beta-pregnane-3,20-dione | HMDB | | 5beta-Pregnane-11beta,21-diol-3,20-dione | HMDB |
|
|---|
| Chemical Formula | C21H32O4 |
|---|
| Average Molecular Mass | 348.476 g/mol |
|---|
| Monoisotopic Mass | 348.230 g/mol |
|---|
| CAS Registry Number | 566-01-8 |
|---|
| IUPAC Name | (1S,2S,7R,10S,11S,15S,17S)-17-hydroxy-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-5-one |
|---|
| Traditional Name | (1S,2S,7R,10S,11S,15S,17S)-17-hydroxy-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-5-one |
|---|
| SMILES | [H][C@@]12CCC(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1([H])[C@@]2([H])CC[C@]2([H])CC(=O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C21H32O4/c1-20-8-7-13(23)9-12(20)3-4-14-15-5-6-16(18(25)11-22)21(15,2)10-17(24)19(14)20/h12,14-17,19,22,24H,3-11H2,1-2H3/t12-,14+,15+,16?,17+,19-,20+,21+/m1/s1 |
|---|
| InChI Key | CTTOFMJLOGMZRN-MGXYISIMSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 21-hydroxysteroids. These are steroids carrying a hydroxyl group at the 21-position of the steroid backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Hydroxysteroids |
|---|
| Direct Parent | 21-hydroxysteroids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Progestogin-skeleton
- 21-hydroxysteroid
- 20-oxosteroid
- Pregnane-skeleton
- 3-oxosteroid
- Oxosteroid
- 11-beta-hydroxysteroid
- 11-hydroxysteroid
- 3-oxo-5-beta-steroid
- Cyclic alcohol
- Alpha-hydroxy ketone
- Ketone
- Secondary alcohol
- Cyclic ketone
- Primary alcohol
- Alcohol
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | - C21 steroids (gluco/mineralocorticoids, progestogins) and derivatives (LMST02030188 )
|
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-0967000000-2bd8bffaffb50939c493 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-004i-1678900000-566d143945a3f4b52be3 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001j-0019000000-0e71fe5c93e16a4cdf43 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01qa-1239000000-f52a428e20d7dd49be68 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01b9-3393000000-deb1166aa0a817378f80 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-4fc0c91330f741f36c93 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00mk-1039000000-1fc49c3cd7bc3ee2d17a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0abi-5093000000-507f39e32c2a16004338 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0009000000-7276daecfbdb64d20c1b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01q9-1779000000-c2eb0110f553b4098951 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0900-6690000000-927e37b4fc2df6d01a4d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-a946d4450827aea0df54 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014j-0029000000-234879f9f1780aa219db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kb-1096000000-ee3717ee0bd77d2944ee | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0006757 |
|---|
| FooDB ID | FDB024061 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 24850104 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 44263339 |
|---|
| Kegg Compound ID | C05475 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|