| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 07:19:38 UTC |
|---|
| Update Date | 2016-11-09 01:21:27 UTC |
|---|
| Accession Number | CHEM036218 |
|---|
| Identification |
|---|
| Common Name | 3-Carboxy-1-hydroxypropylthiamine diphosphate |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
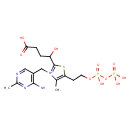
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-(3-Carboxy-1-hydroxypropyl)thiamine diphosphate | ChEBI | | 3-Carboxy-1-hydroxypropyl-THPP | ChEBI | | Succinate semialdehyde-thiamin diphosphate | Kegg | | 2-(3-Carboxy-1-hydroxypropyl)thiamine diphosphoric acid | Generator | | Succinic acid semialdehyde-thiamin diphosphoric acid | Generator | | 3-Carboxy-1-hydroxypropylthiamine diphosphoric acid | Generator | | 3-[(4-Amino-2-methylpyrimidin-5-yl)methyl]-2-(3-carboxy-1-hydroxypropyl)-5-(2-diphosphoethyl)-4-methyl-1,3-thiazol-3-ium | HMDB |
|
|---|
| Chemical Formula | C16H25N4O10P2S |
|---|
| Average Molecular Mass | 527.403 g/mol |
|---|
| Monoisotopic Mass | 527.077 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-2-(3-carboxy-1-hydroxypropyl)-5-(2-{[hydroxy(phosphonooxy)phosphoryl]oxy}ethyl)-4-methyl-1,3-thiazol-3-ium |
|---|
| Traditional Name | 3-carboxy-1-hydroxypropyl-thpp |
|---|
| SMILES | CC1=C(CCOP(O)(=O)OP(O)(O)=O)SC(C(O)CCC(O)=O)=[N+]1CC1=C(N)N=C(C)N=C1 |
|---|
| InChI Identifier | InChI=1S/C16H24N4O10P2S/c1-9-13(5-6-29-32(27,28)30-31(24,25)26)33-16(12(21)3-4-14(22)23)20(9)8-11-7-18-10(2)19-15(11)17/h7,12,21H,3-6,8H2,1-2H3,(H5-,17,18,19,22,23,24,25,26,27,28)/p+1 |
|---|
| InChI Key | ZWUKRGPVMMTMAF-UHFFFAOYSA-O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as thiamine phosphates. These are thiamine derivatives in which the hydroxyl group of the ethanol moiety is substituted by a phosphate group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazines |
|---|
| Sub Class | Pyrimidines and pyrimidine derivatives |
|---|
| Direct Parent | Thiamine phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Thiamine-phosphate
- Organic pyrophosphate
- 2,4,5-trisubstituted 1,3-thiazole
- Aminopyrimidine
- Monoalkyl phosphate
- Organic phosphoric acid derivative
- Imidolactam
- Phosphoric acid ester
- Alkyl phosphate
- Azole
- Heteroaromatic compound
- Thiazole
- Amino acid or derivatives
- Amino acid
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Azacycle
- Organonitrogen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Amine
- Alcohol
- Organic nitrogen compound
- Carbonyl group
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Primary amine
- Aromatic alcohol
- Organic cation
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0ki2-8853930000-2d20db61c35c735c6726 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0002-8080197000-6f52c57ca3a187968f4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-055e-0246950000-bac6074dcea868b654a7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-4319200000-b9cec10cb173f2d20023 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dj-1962000000-76c3988b185ec2f13f69 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05di-0300960000-6d763b4c58b5627dbbe3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004j-0205910000-63d4b7f4c17e449a6f57 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-2937100000-ab1d8c9956ba46638067 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0006744 |
|---|
| FooDB ID | FDB024054 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 389531 |
|---|
| ChEBI ID | 1463 |
|---|
| PubChem Compound ID | 440649 |
|---|
| Kegg Compound ID | C05381 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | ECMDB06744 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|