| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 07:18:38 UTC |
|---|
| Update Date | 2016-11-09 01:21:27 UTC |
|---|
| Accession Number | CHEM036196 |
|---|
| Identification |
|---|
| Common Name | Tri-N-acetylchitotriose |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
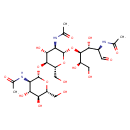
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (g1CNAC)3 | HMDB | | N,N',n''-triacetyl chitotriose | HMDB | | N,N',n''-triacetylchitintriose | HMDB | | N,N',n''-triacetylchitotriose | HMDB | | N-Acetylchitotriose | HMDB | | Tri(N-acetyl-D-glucosamine) | HMDB | | Tris(N-acetylglucosamine) | HMDB | | TriGlcNAc | MeSH, HMDB | | Tri-glcnac | MeSH, HMDB | | O-2-acetamido-2-Deoxy-beta-D-glucopyranosyl-(1-4)-O-2-acetamido-2-deoxy-beta-D-glucopyranosyl-(1-4)-2-acetamido-2-deoxy-D-glucopyranose | MeSH, HMDB | | N,N',n''- triacetyl chitotriose | MeSH, HMDB | | N-[(2R,3R,4S,5R)-4-{[(2S,3R,4R,5S,6R)-5-{[(2S,3R,4R,5S,6R)-4,5-dihydroxy-3-[(1-hydroxyethylidene)amino]-6-(hydroxymethyl)oxan-2-yl]oxy}-4-hydroxy-3-[(1-hydroxyethylidene)amino]-6-(hydroxymethyl)oxan-2-yl]oxy}-3,5,6-trihydroxy-1-oxohexan-2-yl]ethanimidate | Generator, HMDB |
|
|---|
| Chemical Formula | C24H41N3O16 |
|---|
| Average Molecular Mass | 627.593 g/mol |
|---|
| Monoisotopic Mass | 627.249 g/mol |
|---|
| CAS Registry Number | 38864-21-0 |
|---|
| IUPAC Name | N-[(2R,3R,4S,5R)-4-{[(2S,3R,4R,5S,6R)-3-acetamido-5-{[(2S,3R,4R,5S,6R)-3-acetamido-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-4-hydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3,5,6-trihydroxy-1-oxohexan-2-yl]acetamide |
|---|
| Traditional Name | N-[(2R,3R,4S,5R)-4-{[(2S,3R,4R,5S,6R)-3-acetamido-5-{[(2S,3R,4R,5S,6R)-3-acetamido-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-4-hydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3,5,6-trihydroxy-1-oxohexan-2-yl]acetamide |
|---|
| SMILES | CC(=O)N[C@@H](C=O)[C@@H](O)[C@H](O[C@@H]1O[C@H](CO)[C@@H](O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2NC(C)=O)[C@H](O)[C@H]1NC(C)=O)[C@H](O)CO |
|---|
| InChI Identifier | InChI=1S/C24H41N3O16/c1-8(32)25-11(4-28)17(36)21(12(35)5-29)42-24-16(27-10(3)34)20(39)22(14(7-31)41-24)43-23-15(26-9(2)33)19(38)18(37)13(6-30)40-23/h4,11-24,29-31,35-39H,5-7H2,1-3H3,(H,25,32)(H,26,33)(H,27,34)/t11-,12+,13+,14+,15+,16+,17+,18+,19+,20+,21+,22+,23-,24-/m0/s1 |
|---|
| InChI Key | LRDDKCYRFNJZBX-WHFMPQCRSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acyl-alpha-hexosamines. These are carbohydrate derivatives containing a hexose moiety in which the oxygen atom is replaced by an n-acyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | N-acyl-alpha-hexosamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acyl-alpha-hexosamine
- Disaccharide
- Glycosyl compound
- O-glycosyl compound
- Beta-hydroxy aldehyde
- Oxane
- Acetamide
- Carboxamide group
- Secondary carboxylic acid amide
- Secondary alcohol
- Acetal
- Carboxylic acid derivative
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Hydrocarbon derivative
- Organic oxide
- Alcohol
- Organopnictogen compound
- Carbonyl group
- Aldehyde
- Organonitrogen compound
- Primary alcohol
- Organic nitrogen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-08fr-9320262000-8f74509cfc7176a3b286 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-03e9-9400108000-78822546ff9e0663f6cf | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_7) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_8) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_9) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_10) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_11) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_12) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_7) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_8) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_9) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_10) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_11) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_12) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0409-0020297000-3e381d44029510d6192f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0zfr-9831321000-6417feed4e9c6e3c947e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0wml-9753231000-54315c61ff2b0642359a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03nl-3391622000-c2c2ac1f7035756cbf4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0pi0-8795854000-dce4dbb44ac6bd6d82b2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0zmi-6369100000-d5e0054ef614e34fb0d4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a59-2100193000-e537f7d376788ee65f4c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9012350000-fd8f926b7f5600730142 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006x-9022330000-522dc7c3e82c39b6111f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0pdi-0140394000-05c754d36f4aa39391a2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0l7i-9540281000-d628e28f3d9d0a8da018 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001r-9310000000-7bc3bc404954560b5788 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0006698 |
|---|
| FooDB ID | FDB024030 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 110328 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 123774 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Singh, Suddham; Packwood, John; Samuel, Christopher J.; Critchley, P.; Crout, David H. G. Glycosidase-catalyzed oligosaccharide synthesis: preparation of N-acetylchito-oligosaccharides using the b-N-acetylhexosaminidase of Aspergillus oryzae. Carbohydrate Research (1995), 279 293-305. | | 2. Singh, Suddham; Packwood, John; Samuel, Christopher J.; Critchley, P.; Crout, David H. G. Glycosidase-catalyzed oligosaccharide synthesis: preparation of N-acetylchito-oligosaccharides using the b-N-acetylhexosaminidase of Aspergillus oryzae. Carbohydrate Research (1995), 279 293-305. | | 3. Prieels JP, Dolmans M, Schindler M, Sharon N: The binding of glycoconjugates to human-milk D-galactosyltransferase. Eur J Biochem. 1976 Jul 15;66(3):579-82. | | 4. Silano M, De Vincenzi M: Bioactive antinutritional peptides derived from cereal prolamins: a review. Nahrung. 1999 Jun;43(3):175-84. |
|
|---|