| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 07:16:51 UTC |
|---|
| Update Date | 2016-11-09 01:21:26 UTC |
|---|
| Accession Number | CHEM036167 |
|---|
| Identification |
|---|
| Common Name | Sialyl Lewis(a) pentasaccharide |
|---|
| Class | Small Molecule |
|---|
| Description | Sialyl Lewisa pentasaccharide is a human E-selectin (endothelial adhesion molecule) ligand. The cell adhesion molecule E-selectin, which is expressed on cytokine-stimulated endothelial cells, binds to neutrophils, monocytes, and a subpopulation of memory T-cells. This protein has an established role at the initial stepins the recruitment of leukocytes to sites of inflammation, and there is also much interest in the part that it plays in the hematogenous spread of cancer cells. Interference with selectin binding results in inhibition of the later steps that promote leukocyte extravasation. There is therefore much interest in defining the ligands that support adhesion, as a lead to design of adhesion inhibitors that may serve as drugs for the treatment of disorders of inflammation and for minimizing risk of metastasis during surgical resections of tumors. As predicted from the presence of a lectin-like module at the amino-terminal endof the extracellular domain, E-selectin is a carbohydrate-binding protein. The established ligands for E-selectin include a family of blood group-related fucooligosaccharides that occur as differentiation antigens of granulocytes and monocytes (Lex and 3'-sialyl-Lex) and of epithelial tumors (Lea and 3'-sialyl-Lea). Adhesion to the sialyl antigens is substantially greater than to the asialo analogues (PMID:7507478). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
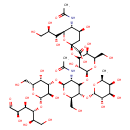
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Sialyl lewisa penta | Generator | | O-(N-Acetyl-alpha-neuraminosyl)-(2->3)-O-beta-D-galactopyranosyl-(1->3)-O-[6-deoxy-alpha-L-galactopyranosyl-(1->4)]-O-2-(acetylamino)-2-deoxy-alpha-D-glucopyranosyl-(1->3)-O-beta-D-galactopyranosyl-(1->4)- D-glucose | HMDB | | O-(N-Acetyl-alpha-neuraminosyl)-(2->3)-O-beta-delta-galactopyranosyl-(1->3)-O-[6-deoxy-alpha-L-galactopyranosyl-(1->4)]-O-2-(acetylamino)-2-deoxy-alpha-delta-glucopyranosyl-(1->3)-O-beta-delta-galactopyranosyl-(1->4)- D-glucose | HMDB | | (2S,4S,5R,6R)-2-{[(2R,3R,5S,6R)-2-{[(2S,3R,5S,6R)-2-{[(2R,3S,4S,5R,6S)-3,5-dihydroxy-2-(hydroxymethyl)-6-{[(2R,3R,4R,5R)-1,2,4,5-tetrahydroxy-6-oxohexan-3-yl]oxy}oxan-4-yl]oxy}-3-[(1-hydroxyethylidene)amino]-6-(hydroxymethyl)-5-{[(2S,3S,4R,5S,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}oxan-4-yl]oxy}-3,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy}-4-hydroxy-5-[(1-hydroxyethylidene)amino]-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylate | Generator, HMDB |
|
|---|
| Chemical Formula | C43H72N2O33 |
|---|
| Average Molecular Mass | 1145.025 g/mol |
|---|
| Monoisotopic Mass | 1144.402 g/mol |
|---|
| CAS Registry Number | 84061-53-0 |
|---|
| IUPAC Name | (2S,4S,5R,6R)-2-{[(2R,3R,5S,6R)-2-{[(2S,3R,5S,6R)-2-{[(2R,3S,4S,5R,6S)-3,5-dihydroxy-2-(hydroxymethyl)-6-{[(2R,3R,4R,5R)-1,2,4,5-tetrahydroxy-6-oxohexan-3-yl]oxy}oxan-4-yl]oxy}-3-acetamido-6-(hydroxymethyl)-5-{[(2S,3S,4R,5S,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}oxan-4-yl]oxy}-3,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy}-5-acetamido-4-hydroxy-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylic acid |
|---|
| Traditional Name | (2S,4S,5R,6R)-2-{[(2R,3R,5S,6R)-2-{[(2S,3R,5S,6R)-2-{[(2R,3S,4S,5R,6S)-3,5-dihydroxy-2-(hydroxymethyl)-6-{[(2R,3R,4R,5R)-1,2,4,5-tetrahydroxy-6-oxohexan-3-yl]oxy}oxan-4-yl]oxy}-3-acetamido-6-(hydroxymethyl)-5-{[(2S,3S,4R,5S,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}oxan-4-yl]oxy}-3,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy}-5-acetamido-4-hydroxy-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylic acid |
|---|
| SMILES | C[C@@H]1O[C@@H](O[C@@H]2[C@@H](CO)O[C@@H](O[C@H]3[C@@H](O)[C@@H](CO)O[C@@H](O[C@H]([C@H](O)CO)[C@H](O)[C@@H](O)C=O)[C@@H]3O)[C@H](NC(C)=O)C2O[C@@H]2O[C@H](CO)[C@H](O)C(O[C@@]3(C[C@H](O)[C@@H](NC(C)=O)[C@@H](O3)[C@H](O)[C@H](O)CO)C(O)=O)[C@H]2O)[C@@H](O)[C@H](O)[C@@H]1O |
|---|
| InChI Identifier | InChI=1S/C43H72N2O33/c1-11-23(58)28(63)29(64)39(69-11)74-33-20(10-51)72-38(76-36-26(61)18(8-49)70-40(30(36)65)73-32(17(57)7-48)24(59)15(55)5-46)22(45-13(3)53)35(33)75-41-31(66)37(27(62)19(9-50)71-41)78-43(42(67)68)4-14(54)21(44-12(2)52)34(77-43)25(60)16(56)6-47/h5,11,14-41,47-51,54-66H,4,6-10H2,1-3H3,(H,44,52)(H,45,53)(H,67,68)/t11-,14-,15-,16+,17+,18+,19+,20+,21+,22+,23+,24+,25+,26-,27-,28+,29-,30+,31+,32+,33+,34+,35?,36-,37?,38-,39-,40-,41-,43-/m0/s1 |
|---|
| InChI Key | DBXMZPZIFJCVQO-RKVJIPOWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acylneuraminic acids. These are neuraminic acids carrying an N-acyl substituent. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | N-acylneuraminic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oligosaccharide
- N-acylneuraminic acid
- Neuraminic acid
- Fatty acyl glycoside
- N-acyl-alpha-hexosamine
- C-glucuronide
- Alkyl glycoside
- C-glycosyl compound
- Glycosyl compound
- O-glycosyl compound
- Ketal
- Beta-hydroxy aldehyde
- Fatty acyl
- Pyran
- Oxane
- Acetamide
- Alpha-hydroxyaldehyde
- Carboxamide group
- Secondary alcohol
- Secondary carboxylic acid amide
- Polyol
- Carboxylic acid derivative
- Carboxylic acid
- Oxacycle
- Acetal
- Monocarboxylic acid or derivatives
- Organoheterocyclic compound
- Organonitrogen compound
- Carbonyl group
- Hydrocarbon derivative
- Primary alcohol
- Aldehyde
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxide
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-003r-1900002023-28fc197cc43f87bca476 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-053l-2702014491-435e0cc2d1f5d7bc4840 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03el-6900043231-8fc411c03905fda0f148 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00c9-6911000000-c83250fa58c2729a22f3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0570-8914001020-ca187e1cc26a4807e349 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a6r-7946400000-9c546e1f7461390fc8f5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-6930000001-9ae35dc643df44c60ae3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0cnm-9610000001-ad9f8b66aa35d0f66dfc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9100010001-92ecb7b05c90a9e18345 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001j-1700000029-efb6bfeb1f8e1e238e55 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01rw-6900000034-d4d89f79d9751f83431b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9800001010-b3e7205f1c992d507b44 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0006605 |
|---|
| FooDB ID | FDB023998 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 59697181 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 53477868 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Yuen CT, Bezouska K, O'Brien J, Stoll M, Lemoine R, Lubineau A, Kiso M, Hasegawa A, Bockovich NJ, Nicolaou KC, et al.: Sulfated blood group Lewis(a). A superior oligosaccharide ligand for human E-selectin. J Biol Chem. 1994 Jan 21;269(3):1595-8. |
|
|---|