| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 07:16:45 UTC |
|---|
| Update Date | 2016-11-09 01:21:26 UTC |
|---|
| Accession Number | CHEM036164 |
|---|
| Identification |
|---|
| Common Name | Sialyllacto-N-tetraose b |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
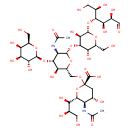
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| LS-Tetrasaccharide b | HMDB | | LSTB | HMDB | | O-(N-Acetyl-alpha-neuraminosyl)-(2->6)-O-[beta-D-galactopyranosyl-(1->3)]-O-2-(acetylamino)-2-deoxy-beta-D-glucopyranosyl-(1->3)-O-beta-D-galactopyranosyl-(1->4)- D-glucose | HMDB | | O-(N-Acetyl-alpha-neuraminosyl)-(2->6)-O-[beta-delta-galactopyranosyl-(1->3)]-O-2-(acetylamino)-2-deoxy-beta-delta-glucopyranosyl-(1->3)-O-beta-delta-galactopyranosyl-(1->4)- D-glucose | HMDB | | (2R,4S,5R,6R)-2-{[(2R,3S,4R,5R,6S)-6-{[(2R,3S,4S,5R,6S)-3,5-dihydroxy-2-(hydroxymethyl)-6-{[(2R,3R,4R,5R)-1,2,4,5-tetrahydroxy-6-oxohexan-3-yl]oxy}oxan-4-yl]oxy}-3-hydroxy-5-[(1-hydroxyethylidene)amino]-4-{[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxan-2-yl]methoxy}-4-hydroxy-5-[(1-hydroxyethylidene)amino]-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylate | Generator, HMDB |
|
|---|
| Chemical Formula | C37H62N2O29 |
|---|
| Average Molecular Mass | 998.884 g/mol |
|---|
| Monoisotopic Mass | 998.344 g/mol |
|---|
| CAS Registry Number | 64003-54-9 |
|---|
| IUPAC Name | (2R,4S,5R,6R)-2-{[(2R,3S,4R,5R,6S)-6-{[(2R,3S,4S,5R,6S)-3,5-dihydroxy-2-(hydroxymethyl)-6-{[(2R,3R,4R,5R)-1,2,4,5-tetrahydroxy-6-oxohexan-3-yl]oxy}oxan-4-yl]oxy}-5-acetamido-3-hydroxy-4-{[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxan-2-yl]methoxy}-5-acetamido-4-hydroxy-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylic acid |
|---|
| Traditional Name | (2R,4S,5R,6R)-2-{[(2R,3S,4R,5R,6S)-6-{[(2R,3S,4S,5R,6S)-3,5-dihydroxy-2-(hydroxymethyl)-6-{[(2R,3R,4R,5R)-1,2,4,5-tetrahydroxy-6-oxohexan-3-yl]oxy}oxan-4-yl]oxy}-5-acetamido-3-hydroxy-4-{[(2R,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxan-2-yl]methoxy}-5-acetamido-4-hydroxy-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylic acid |
|---|
| SMILES | CC(=O)N[C@@H]1[C@@H](O)C[C@@](OC[C@H]2O[C@@H](O[C@H]3[C@@H](O)[C@@H](CO)O[C@@H](O[C@H]([C@H](O)CO)[C@H](O)[C@@H](O)C=O)[C@@H]3O)[C@H](NC(C)=O)[C@@H](O[C@@H]3O[C@H](CO)[C@H](O)[C@H](O)[C@H]3O)[C@@H]2O)(O[C@H]1[C@H](O)[C@H](O)CO)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C37H62N2O29/c1-10(45)38-19-12(47)3-37(36(59)60,68-31(19)22(52)14(49)5-41)61-9-18-25(55)30(66-34-27(57)26(56)23(53)16(7-43)62-34)20(39-11(2)46)33(64-18)67-32-24(54)17(8-44)63-35(28(32)58)65-29(15(50)6-42)21(51)13(48)4-40/h4,12-35,41-44,47-58H,3,5-9H2,1-2H3,(H,38,45)(H,39,46)(H,59,60)/t12-,13-,14+,15+,16+,17+,18+,19+,20+,21+,22+,23-,24-,25+,26-,27+,28+,29+,30+,31+,32-,33-,34-,35-,37+/m0/s1 |
|---|
| InChI Key | SFMRPVLZMVJKGZ-JRZQLMJNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acylneuraminic acids. These are neuraminic acids carrying an N-acyl substituent. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | N-acylneuraminic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oligosaccharide
- N-acylneuraminic acid
- Neuraminic acid
- Fatty acyl glycoside
- N-acyl-alpha-hexosamine
- C-glucuronide
- Alkyl glycoside
- C-glycosyl compound
- Glycosyl compound
- O-glycosyl compound
- Ketal
- Beta-hydroxy aldehyde
- Fatty acyl
- Pyran
- Oxane
- Acetamide
- Alpha-hydroxyaldehyde
- Carboxamide group
- Secondary alcohol
- Secondary carboxylic acid amide
- Polyol
- Carboxylic acid derivative
- Carboxylic acid
- Oxacycle
- Acetal
- Monocarboxylic acid or derivatives
- Organoheterocyclic compound
- Organonitrogen compound
- Carbonyl group
- Hydrocarbon derivative
- Primary alcohol
- Aldehyde
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxide
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01q9-0400000049-979d3487e4d8a30e01d2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-02al-3902043144-b611e89ecda7f2d45a72 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03e9-9810021235-acda66faf61a04af2996 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-05du-3512000019-3cacea2d1164023fdb24 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004r-9722102416-1c718ef0cd4363f278ef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0bvi-9844200000-b65a193acf80f49912ec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-2110000039-b22e912717f2a6ee6bb7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056s-2000000079-df7f949675ad1796045d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-7a551737a6f4c88aacc4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03yi-1200001059-07705dd1c1c9266ac0cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01ot-8500000559-f95c2c86578ee498ef15 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ot-7900010153-f7e3d9eab50b4258d80c | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0006600 |
|---|
| FooDB ID | FDB023994 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30776582 |
|---|
| ChEBI ID | 88450 |
|---|
| PubChem Compound ID | 53477864 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Yamashita, Katsuko; Tachibana, Yoko; Kobata, Akira. Oligosaccharides of human milk: structures of three lacto-N-hexaose derivatives with H-haptenic structure. Archives of Biochemistry and Biophysics (1977), 182(2), 546-55. | | 2. Ravindranath MH, Kelley MC, Jones RC, Amiri AA, Bauer PM, Morton DL: Ratio of IgG:IgM antibodies to sialyl Lewis(x) and GM3 correlates with tumor growth after immunization with melanoma-cell vaccine with different adjuvants in mice. Int J Cancer. 1998 Jan 5;75(1):117-24. | | 3. Kitagawa H, Nakada H, Numata Y, Kurosaka A, Fukui S, Funakoshi I, Kawasaki T, Shimada I, Inagaki F, Yamashina I: Occurrence of tetra- and pentasaccharides with the sialyl-Le(a) structure in human milk. J Biol Chem. 1990 Mar 25;265(9):4859-62. | | 4. Shen Z, Warren CD, Newburg DS: High-performance capillary electrophoresis of sialylated oligosaccharides of human milk. Anal Biochem. 2000 Mar 1;279(1):37-45. | | 5. Kitagawa H, Takaoka M, Nakada H, Fukui S, Funakoshi I, Kawasaki T, Tate S, Inagaki F, Yamashina I: Isolation and structural studies of human milk oligosaccharides that are reactive with a monoclonal antibody MSW 113. J Biochem. 1991 Oct;110(4):598-604. | | 6. Bao Y, Zhu L, Newburg DS: Simultaneous quantification of sialyloligosaccharides from human milk by capillary electrophoresis. Anal Biochem. 2007 Nov 15;370(2):206-14. Epub 2007 Jul 7. | | 7. Asakuma S, Akahori M, Kimura K, Watanabe Y, Nakamura T, Tsunemi M, Arai I, Sanai Y, Urashima T: Sialyl oligosaccharides of human colostrum: changes in concentration during the first three days of lactation. Biosci Biotechnol Biochem. 2007 Jun;71(6):1447-51. |
|
|---|