| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 07:16:30 UTC |
|---|
| Update Date | 2016-11-09 01:21:26 UTC |
|---|
| Accession Number | CHEM036156 |
|---|
| Identification |
|---|
| Common Name | 6-Sialyl-N-acetyllactosamine |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
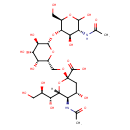
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 6'-Sialyl-N-acetyllactosamine | HMDB | | 62-a-N-Acetylneuraminyl-N-acetyllactosam | HMDB | | 62-alpha-N-Acetylneuraminyl-N-acetyllactosam | HMDB | | 62-alpha-N-Acetylneuraminyl-N-acetyllactosamine | HMDB | | NeuAc alpha,6gal beta1,4gicnac | HMDB | | O-(N-Acetyl-a-neuraminosyl)-(2->6)-O-b-D-galactopyranosyl-(1->4)-2-(acetylamino)-2-deoxy-D-glucose | HMDB | | O-(N-Acetyl-alpha-neuraminosyl)-(2->6)-O-beta-D-galactopyranosyl-(1->4)-2-(acetylamino)-2-deoxy-D-glucose | HMDB | | (2S,4S,5R,6R)-2-{[(2R,3R,4S,5R,6S)-6-{[(2R,3S,4R,5R)-4,6-dihydroxy-5-[(1-hydroxyethylidene)amino]-2-(hydroxymethyl)oxan-3-yl]oxy}-3,4,5-trihydroxyoxan-2-yl]methoxy}-4-hydroxy-5-[(1-hydroxyethylidene)amino]-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylate | Generator, HMDB |
|
|---|
| Chemical Formula | C25H42N2O19 |
|---|
| Average Molecular Mass | 674.603 g/mol |
|---|
| Monoisotopic Mass | 674.238 g/mol |
|---|
| CAS Registry Number | 78969-47-8 |
|---|
| IUPAC Name | (2S,4S,5R,6R)-5-acetamido-2-{[(2R,3R,4S,5R,6S)-6-{[(2R,3S,4R,5R)-5-acetamido-4,6-dihydroxy-2-(hydroxymethyl)oxan-3-yl]oxy}-3,4,5-trihydroxyoxan-2-yl]methoxy}-4-hydroxy-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylic acid |
|---|
| Traditional Name | (2S,4S,5R,6R)-5-acetamido-2-{[(2R,3R,4S,5R,6S)-6-{[(2R,3S,4R,5R)-5-acetamido-4,6-dihydroxy-2-(hydroxymethyl)oxan-3-yl]oxy}-3,4,5-trihydroxyoxan-2-yl]methoxy}-4-hydroxy-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylic acid |
|---|
| SMILES | [H][C@]1(O[C@](C[C@H](O)[C@H]1NC(C)=O)(OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](NC(C)=O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O)C(O)=O)[C@H](O)[C@H](O)CO |
|---|
| InChI Identifier | InChI=1S/C25H42N2O19/c1-7(30)26-13-9(32)3-25(24(40)41,46-21(13)15(34)10(33)4-28)42-6-12-16(35)18(37)19(38)23(44-12)45-20-11(5-29)43-22(39)14(17(20)36)27-8(2)31/h9-23,28-29,32-39H,3-6H2,1-2H3,(H,26,30)(H,27,31)(H,40,41)/t9-,10+,11+,12+,13+,14+,15+,16-,17+,18-,19+,20+,21+,22?,23-,25-/m0/s1 |
|---|
| InChI Key | RPSBVJXBTXEJJG-RAMSCCQBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acylneuraminic acids. These are neuraminic acids carrying an N-acyl substituent. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | N-acylneuraminic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acylneuraminic acid
- Acylaminosugar
- Neuraminic acid
- N-acyl-alpha-hexosamine
- C-glucuronide
- C-glycosyl compound
- Disaccharide
- Glycosyl compound
- O-glycosyl compound
- Ketal
- Pyran
- Oxane
- Acetamide
- Secondary carboxylic acid amide
- Carboxamide group
- Hemiacetal
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Organoheterocyclic compound
- Carboxylic acid
- Carboxylic acid derivative
- Oxacycle
- Acetal
- Polyol
- Organic oxide
- Hydrocarbon derivative
- Alcohol
- Organic nitrogen compound
- Carbonyl group
- Organopnictogen compound
- Organonitrogen compound
- Primary alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0nmi-5235119000-5a6aa8ff9e0838274a4b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_7) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_8) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_9) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_10) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_11) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_12) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_13) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_7) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_8) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_9) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_10) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_11) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0avi-0051009000-92d7600cf12a3e43b8da | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0g4i-2294002000-8d29637be46c1d8fd36f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-022c-4391001000-847f9d4addc27d2b4d8d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00dl-4942473000-7edd91cc87dbeead8022 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05g0-9881203000-a60297bc793daed18eb2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ab9-9641000000-bc983c803272c7ccce3c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0000009000-6626f23b078e7b6b89e8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00lr-0267029000-d0aa4fdac515d5c38553 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f8c-4987000000-3cdf54155f61766e6711 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0020039000-18d48851f2beb9db3adf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-3090022000-8c7f3399bee970f68a60 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052o-9520001000-9d39a2f319fe4cae1e7c | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0006584 |
|---|
| FooDB ID | FDB023986 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 17216384 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 16212424 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|