| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 07:15:46 UTC |
|---|
| Update Date | 2016-11-09 01:21:26 UTC |
|---|
| Accession Number | CHEM036139 |
|---|
| Identification |
|---|
| Common Name | Ecgonine |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | - FooDB Chemicals
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
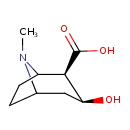
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2R,3S)-3-Hydroxy-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylic acid | HMDB | | 3-b-Hydroxy-2-b-tropanecarboxylic acid | HMDB | | 3-beta-Hydroxy-2-beta-tropanecarboxylic acid | HMDB | | Ekgonin | HMDB | | L-Ecgonine | HMDB | | Ecgonine acetate, (1R-(exo,exo))-isomer | HMDB | | Ecgonine hydrochloride | HMDB | | Ecgonine, (1R-(2-endo,3-exo))-isomer | HMDB |
|
|---|
| Chemical Formula | C9H15NO3 |
|---|
| Average Molecular Mass | 185.220 g/mol |
|---|
| Monoisotopic Mass | 185.105 g/mol |
|---|
| CAS Registry Number | 481-37-8 |
|---|
| IUPAC Name | (2R,3S)-3-hydroxy-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylic acid |
|---|
| Traditional Name | ecgonine |
|---|
| SMILES | CN1C2CCC1[C@H]([C@@H](O)C2)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C9H15NO3/c1-10-5-2-3-6(10)8(9(12)13)7(11)4-5/h5-8,11H,2-4H2,1H3,(H,12,13)/t5?,6?,7-,8+/m0/s1 |
|---|
| InChI Key | PHMBVCPLDPDESM-RLXKETGRSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tropane alkaloids. These are organic compounds containing the nitrogenous bicyclic alkaloid parent N-Methyl-8-azabicyclo[3.2.1]octane. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Tropane alkaloids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Tropane alkaloids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Piperidinecarboxylic acid
- Tropane alkaloid
- Beta-hydroxy acid
- Hydroxy acid
- Piperidine
- N-alkylpyrrolidine
- Cyclic alcohol
- Pyrrolidine
- Amino acid or derivatives
- Amino acid
- Tertiary aliphatic amine
- Tertiary amine
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Azacycle
- Organoheterocyclic compound
- Amine
- Organic oxide
- Organopnictogen compound
- Alcohol
- Carbonyl group
- Organonitrogen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic nitrogen compound
- Organic oxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001j-9200000000-48c3b8ee572b2dc52292 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-006t-9132000000-9a8887ca9dce4b08af81 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014r-0900000000-84cf8248e17a8b226247 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gb9-0900000000-ada9e3b58a4a68bf34fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fk9-3900000000-4fe8aa09d75ccea8cb33 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-007o-0900000000-c8352412d369827945bc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00dl-0900000000-143c9c6dc9126fa654dc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00dl-1900000000-cb49be4d17eb1921ff3e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0900000000-4a6fc2f9a39f6a7a3d44 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kv-5900000000-a2ec3ebcb3ee1f393d9b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dj-9700000000-ac3cdeb9ee66f2c193db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0159-0900000000-dc9636670e82e30cec7c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-007o-1900000000-5cbac175885e0c570c49 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0089-6900000000-987da97e182a8b3e0ba9 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0006548 |
|---|
| FooDB ID | FDB023968 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00002291 |
|---|
| BiGG ID | 2273742 |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Ecgonine |
|---|
| Chemspider ID | 391305 |
|---|
| ChEBI ID | 708641 |
|---|
| PubChem Compound ID | 443003 |
|---|
| Kegg Compound ID | C10858 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | M2MDB004880 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Valente MJ, Henrique R, Vilas-Boas V, Silva R, Bastos Mde L, Carvalho F, Guedes de Pinho P, Carvalho M: Cocaine-induced kidney toxicity: an in vitro study using primary cultured human proximal tubular epithelial cells. Arch Toxicol. 2012 Feb;86(2):249-61. doi: 10.1007/s00204-011-0749-3. Epub 2011 Oct 8. | | 2. Yao D, Shi X, Wang L, Gosnell BA, Chen C: Characterization of differential cocaine metabolism in mouse and rat through metabolomics-guided metabolite profiling. Drug Metab Dispos. 2013 Jan;41(1):79-88. doi: 10.1124/dmd.112.048678. Epub 2012 Oct 3. | | 3. Hezinova V, Aturki Z, Kleparnik K, D'Orazio G, Foret F, Fanali S: Simultaneous analysis of cocaine and its metabolites in urine by capillary electrophoresis-electrospray mass spectrometry using a pressurized liquid junction nanoflow interface. Electrophoresis. 2012 Feb;33(4):653-60. doi: 10.1002/elps.201100410. | | 4. Brim RL, Noon KR, Collins GT, Nichols J, Narasimhan D, Sunahara RK, Woods JH: The ability of bacterial cocaine esterase to hydrolyze cocaine metabolites and their simultaneous quantification using high-performance liquid chromatography-tandem mass spectrometry. Mol Pharmacol. 2011 Dec;80(6):1119-27. doi: 10.1124/mol.111.074534. Epub 2011 Sep 1. | | 5. van Nuijs AL, Abdellati K, Bervoets L, Blust R, Jorens PG, Neels H, Covaci A: The stability of illicit drugs and metabolites in wastewater, an important issue for sewage epidemiology? J Hazard Mater. 2012 Nov 15;239-240:19-23. doi: 10.1016/j.jhazmat.2012.04.030. Epub 2012 Apr 21. | | 6. Gonzalez-Marino I, Quintana JB, Rodriguez I, Sanchez-Mendez N, Cela R: Transformation of cocaine during water chlorination. Anal Bioanal Chem. 2012 Dec;404(10):3135-44. doi: 10.1007/s00216-012-6428-2. Epub 2012 Sep 30. | | 7. Hantson P, Capron A, Wallemacq P: Toxicokinetics of cocaine and metabolites in a body-packer becoming symptomatic. J Forensic Leg Med. 2011 Nov;18(8):385-7. doi: 10.1016/j.jflm.2011.07.004. Epub 2011 Aug 9. | | 8. Saussereau E, Lacroix C, Gaulier JM, Goulle JP: On-line liquid chromatography/tandem mass spectrometry simultaneous determination of opiates, cocainics and amphetamines in dried blood spots. J Chromatogr B Analyt Technol Biomed Life Sci. 2012 Feb 15;885-886:1-7. doi: 10.1016/j.jchromb.2011.11.035. Epub 2011 Dec 2. |
|
|---|