| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 07:15:12 UTC |
|---|
| Update Date | 2016-11-09 01:21:26 UTC |
|---|
| Accession Number | CHEM036123 |
|---|
| Identification |
|---|
| Common Name | 2,3-Diketo-L-gulonate |
|---|
| Class | Small Molecule |
|---|
| Description | 2,3-Diketo-L-gulonate is an intermediate in Ascorbate and aldarate metabolism. 2,3-Diketo-L-gulonate is produced from Dehydroascorbate and then converted to L-Xylonate via the enzyme Lyases (EC 4.1.1.-). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
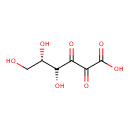
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,3-Diketo-L-gulonic acid | Generator | | (4R,5S)-4,5,6-Trihydroxy-2,3-dioxohexanoate | HMDB | | (4R,5S)-4,5,6-Trihydroxy-2,3-dioxohexanoic acid | HMDB | | 2,3-diketo-L Gulonic acid | HMDB | | 2,3-Diketogulonic acid | HMDB | | Diketogulonic acid | HMDB | | L-threo-2,3-Hexodiulosonic acid | HMDB | | L-threo-hexo-2,3-Diulosonic acid | HMDB |
|
|---|
| Chemical Formula | C6H8O7 |
|---|
| Average Molecular Mass | 192.124 g/mol |
|---|
| Monoisotopic Mass | 192.027 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (5S)-4,5,6-trihydroxy-2,3-dioxohexanoic acid |
|---|
| Traditional Name | (5S)-4,5,6-trihydroxy-2,3-dioxohexanoic acid |
|---|
| SMILES | OC[C@H](O)C(O)C(=O)C(=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C6H8O7/c7-1-2(8)3(9)4(10)5(11)6(12)13/h2-3,7-9H,1H2,(H,12,13)/t2-,3?/m0/s1 |
|---|
| InChI Key | GJQWCDSAOUMKSE-SCQFTWEKSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sugar acids and derivatives. Sugar acids and derivatives are compounds containing a saccharide unit which bears a carboxylic acid group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Sugar acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hexose monosaccharide
- Medium-chain keto acid
- Beta-keto acid
- Sugar acid
- Acyloin
- Alpha-keto acid
- Alpha-diketone
- Beta-hydroxy ketone
- Keto acid
- Monosaccharide
- Alpha-hydroxy ketone
- Ketone
- Secondary alcohol
- Carboxylic acid
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Polyol
- Hydrocarbon derivative
- Carbonyl group
- Alcohol
- Organic oxide
- Primary alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0nn9-9200000000-1cbed9b2b56caa085c4a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-02or-7339800000-1615efb2d0feaa5735ea | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004l-1900000000-1d6c68c73855ddcddb54 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-08mi-9500000000-c792fe4653b44e9ab372 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ab9-9000000000-16a4d7310299656e7744 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0g4l-9700000000-6d882e161ed0ab1a7656 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4r-9300000000-c5167767732d60eead4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-f6c0690482bc1a940237 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01c0-6900000000-f68a48e03dbb51e87373 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-11ou-9500000000-f51f8802520502229315 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0btd-9000000000-b607173f6a4870d24b5f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-005j-7900000000-095b22b39dbcc6d9fa05 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0pb9-9200000000-05380c64aede8d94e31b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-84c78a8bd0634d2dfffc | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0006511 |
|---|
| FooDB ID | FDB023950 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | 45750 |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35015997 |
|---|
| ChEBI ID | 15622 |
|---|
| PubChem Compound ID | 53477844 |
|---|
| Kegg Compound ID | C04575 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|