| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 07:11:46 UTC |
|---|
| Update Date | 2016-11-09 01:21:25 UTC |
|---|
| Accession Number | CHEM036057 |
|---|
| Identification |
|---|
| Common Name | N-Acetylneuraminate 9-phosphate |
|---|
| Class | Small Molecule |
|---|
| Description | N-Acetylneuraminate 9-phosphate is an intermediate in Aminosugars metabolism. N-Acetylneuraminate 9-phosphate is the 4th to last step in the synthesis of colominate and is converted from N-Acetyl-D-mannosamine-6-phosphate via the enzyme N-Acylneuraminate-9-phosphate synthase (EC 2.5.1.57). It is then converted to N-Acetylneuraminate via the enzyme N-acylneuraminate-9-phosphatase(EC 3.1.3.29). |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
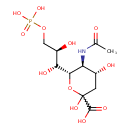
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| N-Acetylneuraminic acid 9-phosphoric acid | Generator |
|
|---|
| Chemical Formula | C11H20NO12P |
|---|
| Average Molecular Mass | 389.250 g/mol |
|---|
| Monoisotopic Mass | 389.072 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (4R,5S,6S)-6-[(1R,2R)-1,2-dihydroxy-3-(phosphonooxy)propyl]-5-acetamido-2,4-dihydroxyoxane-2-carboxylic acid |
|---|
| Traditional Name | (4R,5S,6S)-6-[(1R,2R)-1,2-dihydroxy-3-(phosphonooxy)propyl]-5-acetamido-2,4-dihydroxyoxane-2-carboxylic acid |
|---|
| SMILES | CC(=O)N[C@H]1[C@H](O)CC(O)(O[C@@H]1[C@H](O)[C@H](O)COP(O)(O)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C11H20NO12P/c1-4(13)12-7-5(14)2-11(19,10(17)18)24-9(7)8(16)6(15)3-23-25(20,21)22/h5-9,14-16,19H,2-3H2,1H3,(H,12,13)(H,17,18)(H2,20,21,22)/t5-,6-,7+,8-,9+,11?/m1/s1 |
|---|
| InChI Key | SQMNIXJSBCSNCI-KHLKYKDFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acylneuraminic acids. These are neuraminic acids carrying an N-acyl substituent. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | N-acylneuraminic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acylneuraminic acid
- Neuraminic acid
- C-glucuronide
- C-glycosyl compound
- Glycosyl compound
- Monoalkyl phosphate
- Alpha-hydroxy acid
- Organic phosphoric acid derivative
- Hydroxy acid
- Oxane
- Phosphoric acid ester
- Alkyl phosphate
- Pyran
- Acetamide
- Secondary carboxylic acid amide
- Carboxamide group
- Secondary alcohol
- Hemiacetal
- Carboxylic acid derivative
- Carboxylic acid
- Oxacycle
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Alcohol
- Hydrocarbon derivative
- Carbonyl group
- Organic nitrogen compound
- Organopnictogen compound
- Organonitrogen compound
- Organic oxide
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0007-9743000000-b73738966b1be90e21d5 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-03di-9540238000-9bc6425495a2d9e98f2b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0umj-4494000000-0fa3501a59ff2e606f0a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004r-9000000000-abb61d928b00d92157c7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-9e6e329e3f59fa84a900 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-4009000000-d6b4f4b8476c8d6a4a36 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9011000000-c3431ad5859428c34f7f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-bc034ee0d5c885fabb67 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dl-1139000000-31a61c834ec1e7590647 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dm-6798000000-0e71876811a2de198680 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ow-9360000000-c2de932aecb7f5fddf22 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dl-0069000000-edb6d11a2180e45d1a91 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0597-4396000000-f925a0977a45095edef5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000y-9510000000-0b4a7128a16fcd8e1733 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0006268 |
|---|
| FooDB ID | FDB023867 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | 47736 |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30776539 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 53477814 |
|---|
| Kegg Compound ID | C06241 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|