| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 07:10:32 UTC |
|---|
| Update Date | 2016-11-09 01:21:25 UTC |
|---|
| Accession Number | CHEM036042 |
|---|
| Identification |
|---|
| Common Name | 24-Hydroxycalcitriol |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
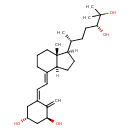
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1alpha,24R,25(OH)3D3 | ChEBI | | 1alpha,24R,25-Trihydroxycholecalciferol | ChEBI | | 1alpha,24R,25-Trihydroxyvitamin D3 | ChEBI | | 1a,24R,25(OH)3D3 | Generator | | 1Α,24R,25(OH)3D3 | Generator | | 1a,24R,25-Trihydroxycholecalciferol | Generator | | 1Α,24R,25-trihydroxycholecalciferol | Generator | | 1a,24R,25-Trihydroxyvitamin D3 | Generator | | 1Α,24R,25-trihydroxyvitamin D3 | Generator | | (1-alpha,3-beta,5Z,7E,24R)-10-Secocholesta-5,7,10(19)-triene-1,3,24,25-tetrol | HMDB | | (1-alpha,3-beta,5Z,7E,24R)-9,10-Secocholesta-5,7,10(19)-triene-1,3,24,25-tetrol | HMDB | | (24R)-24-Hydroxycalcitriol | HMDB | | 1-alpha,24(R),25-Trihydroxyvitamin D3 | HMDB | | 1-alpha,24R,25-Trihydroxyvitamin D3 | HMDB | | Calcitetrol | HMDB | | ro 21-7729 | HMDB |
|
|---|
| Chemical Formula | C27H44O4 |
|---|
| Average Molecular Mass | 432.636 g/mol |
|---|
| Monoisotopic Mass | 432.324 g/mol |
|---|
| CAS Registry Number | 56142-94-0 |
|---|
| IUPAC Name | (1R,3S,5Z)-5-{2-[(1R,3aS,4E,7aR)-1-[(2R,5R)-5,6-dihydroxy-6-methylheptan-2-yl]-7a-methyl-octahydro-1H-inden-4-ylidene]ethylidene}-4-methylidenecyclohexane-1,3-diol |
|---|
| Traditional Name | calcitetrol |
|---|
| SMILES | C[C@H](CC[C@@H](O)C(O)(C)C)[C@@]1([H])CC[C@@]2([H])\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |
|---|
| InChI Identifier | InChI=1S/C27H44O4/c1-17(8-13-25(30)26(3,4)31)22-11-12-23-19(7-6-14-27(22,23)5)9-10-20-15-21(28)16-24(29)18(20)2/h9-10,17,21-25,28-31H,2,6-8,11-16H2,1,3-5H3/b19-9+,20-10-/t17-,21-,22-,23+,24+,25-,27-/m1/s1 |
|---|
| InChI Key | WFZKUWGUJVKMHC-UKBUZQLGSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as vitamin d and derivatives. Vitamin D and derivatives are compounds containing a secosteroid backbone, usually secoergostane or secocholestane. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Vitamin D and derivatives |
|---|
| Direct Parent | Vitamin D and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Triterpenoid
- Tertiary alcohol
- Cyclic alcohol
- Secondary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-8019800000-bd957cd71fdc43e3cf94 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-001i-2212039000-ff7c0fc06a74f042ea4e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014j-0119800000-971c62e3a9b445085374 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002b-0329200000-87fe5af08f7d613843ad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ta-1249100000-e02dd2ffb8dcfe6ac44d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0001900000-1a4a0abd4a0f9c808d15 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03e9-0006900000-f4091fa625ee0575ca96 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00y0-9005200000-f5bbc4087082427238c7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0001900000-e16e6b947da6d8aa4af1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01x0-4404900000-13694b4cd0190a34af95 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-056s-3409500000-aa6bf641780066a0b0e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0229500000-9dce7354a42f6ad2e96c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05mt-2269100000-aa636e01f91f796635dc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dr-2950000000-8a96dbb2c0ff0897a2bd | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0006228 |
|---|
| FooDB ID | FDB023848 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | 2290333 |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4949843 |
|---|
| ChEBI ID | 47799 |
|---|
| PubChem Compound ID | 6446280 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. DeLuca HF: The kidney as an endocrine organ involved in the function of vitamin D. Am J Med. 1975 Jan;58(1):39-47. | | 2. Akeno N, Saikatsu S, Kawane T, Horiuchi N: Mouse vitamin D-24-hydroxylase: molecular cloning, tissue distribution, and transcriptional regulation by 1alpha,25-dihydroxyvitamin D3. Endocrinology. 1997 Jun;138(6):2233-40. | | 3. Erben RG, Bante U, Birner H, Stangassinger M: Prophylactic effects of 1,24,25-trihydroxyvitamin D3 on ovariectomy-induced cancellous bone loss in the rat. Calcif Tissue Int. 1997 May;60(5):434-40. |
|
|---|