| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 07:09:30 UTC |
|---|
| Update Date | 2016-11-09 01:21:25 UTC |
|---|
| Accession Number | CHEM036014 |
|---|
| Identification |
|---|
| Common Name | Indolylacryloylglycine |
|---|
| Class | Small Molecule |
|---|
| Description | Indolylacryloylglycine, also known as IAG or indoleacrylic glycine, belongs to the class of organic compounds known as n-acyl-alpha amino acids. N-acyl-alpha amino acids are compounds containing an alpha amino acid which bears an acyl group at its terminal nitrogen atom. Indolylacryloylglycine is possibly soluble (in water) and a moderately basic compound (based on its pKa). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
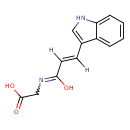
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| IAG | HMDB | | Indole-3-acryloyl-glycine | HMDB | | Indoleacrylic glycine | HMDB | | Indolyl acryloylglycine | HMDB | | N-(3-(1-indol-3-yl)-1-oxo-2-Propenyl)glycine | HMDB | | Indolylacryloylglycine, (e)-isomer | MeSH, HMDB | | Indolyl-3-acryloylglycine | MeSH, HMDB | | 2-{[(2E)-1-hydroxy-3-(1H-indol-3-yl)prop-2-en-1-ylidene]amino}acetate | Generator, HMDB | | Indolylacryloylglycine | MeSH |
|
|---|
| Chemical Formula | C13H12N2O3 |
|---|
| Average Molecular Mass | 244.246 g/mol |
|---|
| Monoisotopic Mass | 244.085 g/mol |
|---|
| CAS Registry Number | 3475-68-1 |
|---|
| IUPAC Name | 2-{[(2E)-1-hydroxy-3-(1H-indol-3-yl)prop-2-en-1-ylidene]amino}acetic acid |
|---|
| Traditional Name | {[(2E)-1-hydroxy-3-(1H-indol-3-yl)prop-2-en-1-ylidene]amino}acetic acid |
|---|
| SMILES | OC(=O)CNC(=O)\C=C\C1=CNC2=CC=CC=C12 |
|---|
| InChI Identifier | InChI=1S/C13H12N2O3/c16-12(15-8-13(17)18)6-5-9-7-14-11-4-2-1-3-10(9)11/h1-7,14H,8H2,(H,15,16)(H,17,18)/b6-5+ |
|---|
| InChI Key | DUIFVCFSAWHIOD-AATRIKPKSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acyl-alpha amino acids. N-acyl-alpha amino acids are compounds containing an alpha amino acid which bears an acyl group at its terminal nitrogen atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | N-acyl-alpha amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acyl-alpha-amino acid
- Indole
- Indole or derivatives
- Substituted pyrrole
- Benzenoid
- Pyrrole
- Heteroaromatic compound
- Secondary carboxylic acid amide
- Carboxamide group
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Azacycle
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Carbonyl group
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-1910000000-af9421f979b84f7c1d21 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00dj-5920000000-34a6af95e2fa1e07e867 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002b-4590000000-bfd50d5180c6bd14c6cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-056r-9710000000-8b4ac64ee5199a960b5e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-056u-7900000000-12988d991d4fb8d3f60c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0190000000-4a969f2be20f7b81f7fb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006x-6890000000-d7bb4b025f6e9caddfc7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05fu-9400000000-0ac0d883ab78f58631e6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-006x-0960000000-27152d1fd1bb75d52117 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006x-2920000000-95b20ae472699500646a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-0900000000-e1ada5cb92852f044560 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002b-0190000000-d2eeedccf34de3bbcbb5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-006x-0910000000-19365eb3817e6e25ff6f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-0900000000-e7f97c6d0f9554f2ee7a | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0006005 |
|---|
| FooDB ID | FDB023799 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4521424 |
|---|
| ChEBI ID | 145761 |
|---|
| PubChem Compound ID | 5370648 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Xi X, Kwok LY, Wang Y, Ma C, Mi Z, Zhang H: Ultra-performance liquid chromatography-quadrupole-time of flight mass spectrometry MS(E)-based untargeted milk metabolomics in dairy cows with subclinical or clinical mastitis. J Dairy Sci. 2017 Jun;100(6):4884-4896. doi: 10.3168/jds.2016-11939. Epub 2017 Mar 23. | | 2. Bull G, Shattock P, Whiteley P, Anderson R, Groundwater PW, Lough JW, Lees G: Indolyl-3-acryloylglycine (IAG) is a putative diagnostic urinary marker for autism spectrum disorders. Med Sci Monit. 2003 Oct;9(10):CR422-5. | | 3. Wright B, Brzozowski AM, Calvert E, Farnworth H, Goodall DM, Holbrook I, Imrie G, Jordan J, Kelly A, Miles J, Smith R, Town J: Is the presence of urinary indolyl-3-acryloylglycine associated with autism spectrum disorder? Dev Med Child Neurol. 2005 Mar;47(3):190-2. | | 4. Anderson RJ, Bendell DJ, Garnett I, Groundwater PW, Lough WJ, Mills MJ, Savery D, Shattock PE: Identification of indolyl-3-acryloylglycine in the urine of people with autism. J Pharm Pharmacol. 2002 Feb;54(2):295-8. |
|
|---|