| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 06:32:28 UTC |
|---|
| Update Date | 2016-11-09 01:21:22 UTC |
|---|
| Accession Number | CHEM035784 |
|---|
| Identification |
|---|
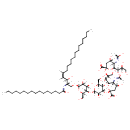
| Common Name | Ganglioside GD2 (d18:1/12:0) |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2S,4S,5R)-2-{[(1S,2R)-1-[(3R,4S,6S)-6-carboxy-6-{[(2S,3R,4S,5S,6R)-2-{[(2R,3S,4R,6R)-4,5-dihydroxy-6-{[(2S,3R,4Z)-3-hydroxy-2-[(1-hydroxyhexadecylidene)amino]octadec-4-en-1-yl]oxy}-2-(hydroxymethyl)oxan-3-yl]oxy}-3,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy}-4-hydroxy-3-[(1-hydroxyethylidene)amino]oxan-2-yl]-1,3-dihydroxypropan-2-yl]oxy}-4-hydroxy-5-[(1-hydroxyethylidene)amino]-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylate | Generator |
|
|---|
| Chemical Formula | Not Available |
|---|
| Average Molecular Mass | Not Available |
|---|
| Monoisotopic Mass | Not Available |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (2S,4S,5R)-2-{[(1S,2R)-1-[(3R,4S,6S)-6-carboxy-6-{[(2S,3R,4S,5S,6R)-2-{[(2R,3S,4R,6R)-4,5-dihydroxy-6-{[(2S,3R,4Z)-3-hydroxy-2-[(1-hydroxyhexadecylidene)amino]octadec-4-en-1-yl]oxy}-2-(hydroxymethyl)oxan-3-yl]oxy}-3,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy}-4-hydroxy-3-[(1-hydroxyethylidene)amino]oxan-2-yl]-1,3-dihydroxypropan-2-yl]oxy}-4-hydroxy-5-[(1-hydroxyethylidene)amino]-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylic acid |
|---|
| Traditional Name | (2S,4S,5R)-2-{[(1S,2R)-1-[(3R,4S,6S)-6-carboxy-6-{[(2S,3R,4S,5S,6R)-2-{[(2R,3S,4R,6R)-4,5-dihydroxy-6-{[(2S,3R,4Z)-3-hydroxy-2-[(1-hydroxyhexadecylidene)amino]octadec-4-en-1-yl]oxy}-2-(hydroxymethyl)oxan-3-yl]oxy}-3,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy}-4-hydroxy-3-[(1-hydroxyethylidene)amino]oxan-2-yl]-1,3-dihydroxypropan-2-yl]oxy}-4-hydroxy-5-[(1-hydroxyethylidene)amino]-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylic acid |
|---|
| SMILES | Not Available |
|---|
| InChI Identifier | Not Available |
|---|
| InChI Key | Not Available |
|---|
| Chemical Taxonomy |
|---|
| Classification | Not classified |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-06wc-0311912120-32f156e720b644d83589 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ikl-0500022290-10dd8fb361d03dd87976 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-7598335650-81c8ee9b79c918066bca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-1224911000-d86fc313348770003108 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4r-7259112010-31324b9b288156f6fe96 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-4559001010-837e64a94418538518fa | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|