| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 06:22:37 UTC |
|---|
| Update Date | 2016-11-09 01:21:22 UTC |
|---|
| Accession Number | CHEM035783 |
|---|
| Identification |
|---|
| Common Name | Ganglioside GD3 (d18:1/9Z-18:1) |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
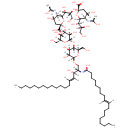
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2S,4S,5R)-2-{[(1S,2R)-1-[(3R,4S,6S)-6-carboxy-6-{[(2S,3R,4S,5S,6R)-2-{[(2R,3S,4R,5R,6R)-4,5-dihydroxy-6-{[(2S,3R,4E)-3-hydroxy-2-{[(9E)-1-hydroxyoctadec-9-en-1-ylidene]amino}octadec-4-en-1-yl]oxy}-2-(hydroxymethyl)oxan-3-yl]oxy}-3,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy}-4-hydroxy-3-[(1-hydroxyethylidene)amino]oxan-2-yl]-1,3-dihydroxypropan-2-yl]oxy}-4-hydroxy-5-[(1-hydroxyethylidene)amino]-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylate | Generator |
|
|---|
| Chemical Formula | C70H123N3O29 |
|---|
| Average Molecular Mass | 1470.746 g/mol |
|---|
| Monoisotopic Mass | 1469.824 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (2S,4S,5R)-2-{[(1S,2R)-1-[(3R,4S,6S)-6-carboxy-6-{[(2S,3R,4S,5S,6R)-2-{[(2R,3S,4R,5R,6R)-4,5-dihydroxy-6-{[(2S,3R,4E)-3-hydroxy-2-{[(9E)-1-hydroxyoctadec-9-en-1-ylidene]amino}octadec-4-en-1-yl]oxy}-2-(hydroxymethyl)oxan-3-yl]oxy}-3,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy}-4-hydroxy-3-[(1-hydroxyethylidene)amino]oxan-2-yl]-1,3-dihydroxypropan-2-yl]oxy}-4-hydroxy-5-[(1-hydroxyethylidene)amino]-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylic acid |
|---|
| Traditional Name | (2S,4S,5R)-2-{[(1S,2R)-1-[(3R,4S,6S)-6-carboxy-6-{[(2S,3R,4S,5S,6R)-2-{[(2R,3S,4R,5R,6R)-4,5-dihydroxy-6-{[(2S,3R,4E)-3-hydroxy-2-{[(9E)-1-hydroxyoctadec-9-en-1-ylidene]amino}octadec-4-en-1-yl]oxy}-2-(hydroxymethyl)oxan-3-yl]oxy}-3,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy}-4-hydroxy-3-[(1-hydroxyethylidene)amino]oxan-2-yl]-1,3-dihydroxypropan-2-yl]oxy}-4-hydroxy-5-[(1-hydroxyethylidene)amino]-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylic acid |
|---|
| SMILES | [H]\C(CCCCCCCC)=C(\[H])CCCCCCCC(O)=N[C@@]([H])(CO[C@]1([H])O[C@]([H])(CO)[C@@]([H])(O[C@]2([H])O[C@]([H])(CO)[C@]([H])(O)[C@]([H])(O[C@@]3(C[C@]([H])(O)[C@@]([H])(N=C(C)O)C([H])(O3)[C@]([H])(O)[C@@]([H])(CO)O[C@@]3(C[C@]([H])(O)[C@@]([H])(N=C(C)O)C([H])(O3)[C@]([H])(O)[C@]([H])(O)CO)C(O)=O)C(O)=O)[C@@]2([H])O)[C@]([H])(O)[C@@]1([H])O)[C@]([H])(O)C(\[H])=C(/[H])CCCCCCCCCCCCC |
|---|
| InChI Identifier | InChI=1S/C70H123N3O29/c1-5-7-9-11-13-15-17-19-20-22-24-26-28-30-32-34-52(84)73-44(45(80)33-31-29-27-25-23-21-18-16-14-12-10-8-6-2)41-95-65-59(89)58(88)61(51(40-77)97-65)98-66-60(90)64(56(86)49(38-75)96-66)102-70(68(93)94)36-47(82)54(72-43(4)79)63(101-70)57(87)50(39-76)99-69(67(91)92)35-46(81)53(71-42(3)78)62(100-69)55(85)48(83)37-74/h19-20,31,33,44-51,53-66,74-77,80-83,85-90H,5-18,21-30,32,34-41H2,1-4H3,(H,71,78)(H,72,79)(H,73,84)(H,91,92)(H,93,94)/b20-19+,33-31+/t44-,45+,46-,47-,48+,49+,50+,51+,53+,54+,55+,56-,57+,58+,59+,60+,61+,62?,63?,64-,65+,66-,69+,70-/m0/s1 |
|---|
| InChI Key | MRXGLRVDPJMCHK-YBVMLHCASA-N |
|---|
| Chemical Taxonomy |
|---|
| Classification | Not classified |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-1159-0310910320-6d965e543e1a8a0a0f10 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03mr-0500020390-02ffe25a33d77ca57762 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03g0-6577440950-132fee0eee388c78fb24 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0zfu-1221900100-0b063f2fbebe72a9b511 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4r-7259110210-02a1d419fc4282ffef01 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-4569000110-f2944c41a20117afd2b9 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|