| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:59:24 UTC |
|---|
| Update Date | 2016-11-09 01:21:21 UTC |
|---|
| Accession Number | CHEM035687 |
|---|
| Identification |
|---|
| Common Name | Tetrahydrogestrinone |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
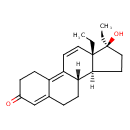
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (17a)-13-Ethyl-17-hydroxy-18,19-dinorpregna-4,9,11-trien-3-one | HMDB | | THG | HMDB | | Tetrahydrogestrinone | MeSH | | 17-HYDROXY-18a-homo-19-nor-17a-pregna-4,9,11-trien-3-one | Generator, HMDB | | 17-HYDROXY-18a-homo-19-nor-17α-pregna-4,9,11-trien-3-one | Generator, HMDB |
|
|---|
| Chemical Formula | C21H28O2 |
|---|
| Average Molecular Mass | 312.446 g/mol |
|---|
| Monoisotopic Mass | 312.209 g/mol |
|---|
| CAS Registry Number | 618903-56-3 |
|---|
| IUPAC Name | (10S,11S,14S,15S)-14,15-diethyl-14-hydroxytetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-1,6,16-trien-5-one |
|---|
| Traditional Name | tetrahydrogestrinone |
|---|
| SMILES | [H][C@@]12CC[C@@](O)(CC)[C@@]1(CC)C=CC1=C3CCC(=O)C=C3CC[C@@]21[H] |
|---|
| InChI Identifier | InChI=1S/C21H28O2/c1-3-20-11-9-17-16-8-6-15(22)13-14(16)5-7-18(17)19(20)10-12-21(20,23)4-2/h9,11,13,18-19,23H,3-8,10,12H2,1-2H3/t18-,19+,20+,21+/m1/s1 |
|---|
| InChI Key | OXHNQTSIKGHVBH-ANULTFPQSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as estrogens and derivatives. These are steroids with a structure containing a 3-hydroxylated estrane. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Estrane steroids |
|---|
| Direct Parent | Estrogens and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Estrogen-skeleton
- 3-oxosteroid
- Hydroxysteroid
- 17-hydroxysteroid
- Oxosteroid
- Cyclohexenone
- Cyclic alcohol
- Tertiary alcohol
- Cyclic ketone
- Ketone
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Alcohol
- Organooxygen compound
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-1190000000-df8a155fb516cec4576a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0a4i-2029000000-895175c474d195f98cc4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01ot-0095000000-8cbb62095c5e14373a79 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01ot-0191000000-e69b0d42106f07a3727c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0umi-1490000000-96c8e821e03405188c49 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0029000000-c002fd210563a9508a67 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0089000000-1b89e06ba01d60280bdd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00lr-0090000000-e424a28e9a87e909dcf0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0009000000-152b659b4891e36b54f3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0029000000-5372cd42b1affac2ac15 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-0091000000-94f3485bb8e82ec323c2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0059000000-a33ccf5f4a2ed5241d04 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0490000000-e755336609970c5c9e52 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00te-1930000000-224c330abfdea1cfae22 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB06870 |
|---|
| HMDB ID | HMDB0004626 |
|---|
| FooDB ID | FDB023385 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | 17H |
|---|
| Wikipedia Link | Tetrahydrogestrinone |
|---|
| Chemspider ID | 5257020 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 6857686 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Labrie, Fernand; Luu-The, Van; Calvo, Ezequiel; Martel, Celine; Cloutier, Julie; Gauthier, Sylvain; Belleau, Pascal; Morissette, Jean; Levesque, Marie-Helene; Labrie, Claude. Tetrahydrogestrinone induces a genomic signature typical of a potent anabolic steroid. J Endocrinol. 2005 Feb;184(2):427-33. Pubmed: 15684350 | | 2. Thevis M, Geyer H, Mareck U, Schanzer W: Screening for unknown synthetic steroids in human urine by liquid chromatography-tandem mass spectrometry. J Mass Spectrom. 2005 Jul;40(7):955-62. | | 3. Handelsman DJ: Designer androgens in sport: when too much is never enough. Sci STKE. 2004 Jul 27;2004(244):pe41. |
|
|---|