| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:59:19 UTC |
|---|
| Update Date | 2016-11-09 01:21:21 UTC |
|---|
| Accession Number | CHEM035685 |
|---|
| Identification |
|---|
| Common Name | 7a-Hydroxydehydroepiandrosterone |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
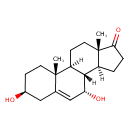
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3beta,7alpha)-3,7-Dihydroxyandrost-5-en-17-one | ChEBI | | 3beta,7alpha-Dihydroxy-5-androstene-17-one | ChEBI | | 3beta,7alpha-Dihydroxyandrost-5-en-17-one | ChEBI | | 7alpha-Hydroxy-dhea | ChEBI | | 7alpha-OH-DHEA | ChEBI | | (3b,7a)-3,7-Dihydroxyandrost-5-en-17-one | Generator | | (3Β,7α)-3,7-dihydroxyandrost-5-en-17-one | Generator | | 3b,7a-Dihydroxy-5-androstene-17-one | Generator | | 3Β,7α-dihydroxy-5-androstene-17-one | Generator | | 3b,7a-Dihydroxyandrost-5-en-17-one | Generator | | 3Β,7α-dihydroxyandrost-5-en-17-one | Generator | | 7a-Hydroxy-dhea | Generator | | 7Α-hydroxy-dhea | Generator | | 7a-OH-DHEA | Generator | | 7Α-OH-dhea | Generator | | (-)-7a-Hydroxydehydroepiandrosterone | HMDB | | (3b,7a)-3,7-Dihydroxy-androst-5-en-17-one | HMDB | | 3b,7a-Dihydroxy-5-androsten-17-one | HMDB | | 3b,7a-Dihydroxy-androst-5-en-17-one | HMDB | | 7-a-OH-DHEA | HMDB | | 7-alpha-OH-DHEA | HMDB | | 7a-Hydroxydehydroisoandrosterone | HMDB | | 7 alpha-Hydroxydehydroepiandrosterone | HMDB | | 7-Hydroxydehydroepiandrosterone, (3beta,7beta)-isomer | HMDB | | 7-Hydroxydehydroepiandrosterone, (3beta)-isomer | HMDB | | 7beta-Hydroxydehydroepiandrosterone | HMDB | | 7beta-OH-DHEA | HMDB | | 7-Hydroxydehydroepiandrosterone | HMDB |
|
|---|
| Chemical Formula | C19H28O3 |
|---|
| Average Molecular Mass | 304.424 g/mol |
|---|
| Monoisotopic Mass | 304.204 g/mol |
|---|
| CAS Registry Number | 53-00-9 |
|---|
| IUPAC Name | (1S,2R,5S,9S,10R,11S,15S)-5,9-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-en-14-one |
|---|
| Traditional Name | 7β-oh-dhea |
|---|
| SMILES | [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])[C@H](O)C=C2C[C@@H](O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C19H28O3/c1-18-7-5-12(20)9-11(18)10-15(21)17-13-3-4-16(22)19(13,2)8-6-14(17)18/h10,12-15,17,20-21H,3-9H2,1-2H3/t12-,13-,14-,15+,17-,18-,19-/m0/s1 |
|---|
| InChI Key | OLPSAOWBSPXZEA-JIEICEMKSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as androgens and derivatives. These are 3-hydroxylated C19 steroid hormones. They are known to favor the development of masculine characteristics. They also show profound effects on scalp and body hair in humans. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Androstane steroids |
|---|
| Direct Parent | Androgens and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Androgen-skeleton
- 3-hydroxy-delta-5-steroid
- 3-hydroxysteroid
- 7-hydroxysteroid
- 3-beta-hydroxysteroid
- 3-beta-hydroxy-delta-5-steroid
- Oxosteroid
- 17-oxosteroid
- Hydroxysteroid
- Delta-5-steroid
- Cyclic alcohol
- Secondary alcohol
- Ketone
- Organic oxygen compound
- Organooxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Alcohol
- Organic oxide
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03g0-0390000000-5b84fc74cc8c3d885c1b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-003r-5204900000-bdb069dd8202b45f9ea3 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-0092000000-003d307a3c381597e38b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ap0-0190000000-7563041720b768705389 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a6u-3190000000-8012f9fad41969d73d46 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0049000000-3459db2d82d83321802f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udr-0098000000-6a1aaf578c3c1719798a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-059f-2090000000-85d4a8001358a115f59b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0009000000-93986d59045cc7fce551 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0019000000-8b3060136e49afe5a336 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uy0-0093000000-e42b11a1c3c059294db3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0069000000-61a09d88b13611d21a89 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0670-1981000000-b0f1410f207bdaec9fa1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05dl-3910000000-4b2efd9d887548375fc0 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0004611 |
|---|
| FooDB ID | FDB023382 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | 7alpha-Hydroxy-DHEA |
|---|
| Chemspider ID | 58963 |
|---|
| ChEBI ID | 712194 |
|---|
| PubChem Compound ID | 65517 |
|---|
| Kegg Compound ID | C18045 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Ishidate, Morizo; Okada, Masashi. Steroids. (1963), 2 pp. JP 38023170 19631031 Showa. CAN 60:12972 AN 1964:12972 | | 2. Ishidate, Morizo; Okada, Masashi. Steroids. (1963), 2 pp. JP 38023170 19631031 Showa. CAN 60:12972 AN 1964:12972 | | 3. Hennebert O, Chalbot S, Alran S, Morfin R: Dehydroepiandrosterone 7alpha-hydroxylation in human tissues: possible interference with type 1 11beta-hydroxysteroid dehydrogenase-mediated processes. J Steroid Biochem Mol Biol. 2007 May;104(3-5):326-33. Epub 2007 Mar 24. | | 4. Robinzon B, Michael KK, Ripp SL, Winters SJ, Prough RA: Glucocorticoids inhibit interconversion of 7-hydroxy and 7-oxo metabolites of dehydroepiandrosterone: a role for 11beta-hydroxysteroid dehydrogenases? Arch Biochem Biophys. 2003 Apr 15;412(2):251-8. | | 5. Dulos J, van der Vleuten MA, Kavelaars A, Heijnen CJ, Boots AM: CYP7B expression and activity in fibroblast-like synoviocytes from patients with rheumatoid arthritis: regulation by proinflammatory cytokines. Arthritis Rheum. 2005 Mar;52(3):770-8. | | 6. Attal-Khemis S, Dalmeyda V, Michot JL, Roudier M, Morfin R: Increased total 7 alpha-hydroxy-dehydroepiandrosterone in serum of patients with Alzheimer's disease. J Gerontol A Biol Sci Med Sci. 1998 Mar;53(2):B125-32. | | 7. https://www.ncbi.nlm.nih.gov/pubmed/?term=12676372 | | 8. https://www.ncbi.nlm.nih.gov/pubmed/?term=14977864 | | 9. https://www.ncbi.nlm.nih.gov/pubmed/?term=15013692 | | 10. https://www.ncbi.nlm.nih.gov/pubmed/?term=15476247 | | 11. https://www.ncbi.nlm.nih.gov/pubmed/?term=15582537 | | 12. https://www.ncbi.nlm.nih.gov/pubmed/?term=15698552 | | 13. https://www.ncbi.nlm.nih.gov/pubmed/?term=15751070 | | 14. https://www.ncbi.nlm.nih.gov/pubmed/?term=15784286 | | 15. https://www.ncbi.nlm.nih.gov/pubmed/?term=16371229 | | 16. https://www.ncbi.nlm.nih.gov/pubmed/?term=16524719 | | 17. https://www.ncbi.nlm.nih.gov/pubmed/?term=16603347 | | 18. https://www.ncbi.nlm.nih.gov/pubmed/?term=16713254 | | 19. https://www.ncbi.nlm.nih.gov/pubmed/?term=16855167 | | 20. https://www.ncbi.nlm.nih.gov/pubmed/?term=17152345 | | 21. https://www.ncbi.nlm.nih.gov/pubmed/?term=17467270 | | 22. https://www.ncbi.nlm.nih.gov/pubmed/?term=17681749 | | 23. https://www.ncbi.nlm.nih.gov/pubmed/?term=18555503 | | 24. https://www.ncbi.nlm.nih.gov/pubmed/?term=20024891 | | 25. https://www.ncbi.nlm.nih.gov/pubmed/?term=20153344 | | 26. https://www.ncbi.nlm.nih.gov/pubmed/?term=20553962 | | 27. https://www.ncbi.nlm.nih.gov/pubmed/?term=24182335 | | 28. https://www.ncbi.nlm.nih.gov/pubmed/?term=26680473 | | 29. https://www.ncbi.nlm.nih.gov/pubmed/?term=26680484 | | 30. https://www.ncbi.nlm.nih.gov/pubmed/?term=28948822 |
|
|---|