| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:58:11 UTC |
|---|
| Update Date | 2016-11-09 01:21:21 UTC |
|---|
| Accession Number | CHEM035663 |
|---|
| Identification |
|---|
| Common Name | Bradykinin |
|---|
| Class | Small Molecule |
|---|
| Description | Bradykinin has been investigated for the basic science and treatment of Hypertension and Diabetes Type 2. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
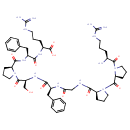
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Arg-pro-pro-gly-phe-ser-pro-phe-arg | ChEBI | | BK | ChEBI | | L-Arg-L-pro-L-pro-gly-L-phe-L-ser-L-pro-L-phe-L-arg | ChEBI | | L-Bradykinin | ChEBI | | RPPGFSPFR | ChEBI | | Callidin I | HMDB | | Kallidin 9 | HMDB | | Kallidin I | HMDB | | L-Arginyl-L-prolyl-L-prolylglycyl-L-phenylalanyl-L-seryl-L-prolyl-L-phenylalanyl-L-arginine | HMDB | | Bradykinin acetate, (9-D-arg)-isomer | HMDB | | Bradykinin hydrochloride | HMDB | | Bradykinin triacetate | HMDB | | Bradykinin, (2-D-pro-7-D-pro)-isomer | HMDB | | Bradykinin, (8-D-phe)-isomer | HMDB | | Arg pro pro gly phe ser pro phe arg | HMDB | | Bradykinin, (1-D-arg)-isomer | HMDB | | Bradykinin, (2-D-pro-3-D-pro-7-D-pro)-isomer | HMDB | | Bradykinin, (3-D-pro-7-D-pro)-isomer | HMDB | | Bradykinin, (5-D-phe)-isomer | HMDB | | Bradykinin, (5-D-phe-8-D-phe)-isomer | HMDB | | Bradykinin, (6-D-ser)-isomer | HMDB | | Bradykinin, (7-D-pro)-isomer | HMDB | | Bradykinin, (9-D-arg)-isomer | HMDB | | Bradykinin diacetate | HMDB | | Bradykinin, (2-D-pro)-isomer | HMDB | | Bradykinin, (3-D-pro)-isomer | HMDB |
|
|---|
| Chemical Formula | C50H73N15O11 |
|---|
| Average Molecular Mass | 1060.209 g/mol |
|---|
| Monoisotopic Mass | 1059.561 g/mol |
|---|
| CAS Registry Number | 58-82-2 |
|---|
| IUPAC Name | (2S)-2-[(2S)-2-{[(2S)-1-[(2S)-2-[(2S)-2-(2-{[(2S)-1-[(2S)-1-[(2S)-2-amino-5-carbamimidamidopentanoyl]pyrrolidine-2-carbonyl]pyrrolidin-2-yl]formamido}acetamido)-3-phenylpropanamido]-3-hydroxypropanoyl]pyrrolidin-2-yl]formamido}-3-phenylpropanamido]-5-carbamimidamidopentanoic acid |
|---|
| Traditional Name | L-bradykinin |
|---|
| SMILES | N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C50H73N15O11/c51-32(16-7-21-56-49(52)53)45(72)65-25-11-20-39(65)47(74)64-24-9-18-37(64)43(70)58-28-40(67)59-34(26-30-12-3-1-4-13-30)41(68)62-36(29-66)46(73)63-23-10-19-38(63)44(71)61-35(27-31-14-5-2-6-15-31)42(69)60-33(48(75)76)17-8-22-57-50(54)55/h1-6,12-15,32-39,66H,7-11,16-29,51H2,(H,58,70)(H,59,67)(H,60,69)(H,61,71)(H,62,68)(H,75,76)(H4,52,53,56)(H4,54,55,57)/t32-,33-,34-,35-,36-,37-,38-,39-/m0/s1 |
|---|
| InChI Key | QXZGBUJJYSLZLT-FDISYFBBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Oligopeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-oligopeptide
- Phenylalanine or derivatives
- Proline or derivatives
- N-acyl-alpha amino acid or derivatives
- N-acyl-l-alpha-amino acid
- N-acyl-alpha-amino acid
- Alpha-amino acid amide
- N-substituted-alpha-amino acid
- Alpha-amino acid or derivatives
- Amphetamine or derivatives
- N-acylpyrrolidine
- Pyrrolidine carboxylic acid or derivatives
- Pyrrolidine-2-carboxamide
- Monocyclic benzene moiety
- Fatty acyl
- Benzenoid
- Fatty amide
- Tertiary carboxylic acid amide
- Pyrrolidine
- Secondary carboxylic acid amide
- Carboxamide group
- Amino acid or derivatives
- Guanidine
- Amino acid
- Azacycle
- Organoheterocyclic compound
- Carboxylic acid
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Monocarboxylic acid or derivatives
- Carboximidamide
- Amine
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Carbonyl group
- Alcohol
- Primary aliphatic amine
- Organic oxide
- Hydrocarbon derivative
- Primary amine
- Primary alcohol
- Organonitrogen compound
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-9605021310-963d37a5c0a6a8c3b581 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00fr-4914021100-da28e55acbc5e55e7615 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9824010200-b5b2c55602c29375ed1a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0ard-9000000008-70e85b5126294f4866f8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4m-8001001009-d8431ee82b28629bac33 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9001111000-9de36ae5c0196bc1ca98 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-9000000000-eb21ccd98d434e431a1e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dl-9000210241-b63ee10d53ba3efc65cb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-022l-9780340000-45c697c4a631655e2811 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-9000000000-6f7d24ea48539f7cd9fb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052f-9000100016-0338af4c69622fd514d8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052g-9003871073-646bd5ef5605a552ce67 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB12126 |
|---|
| HMDB ID | HMDB0004246 |
|---|
| FooDB ID | FDB023350 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Bradykinin |
|---|
| Chemspider ID | 388341 |
|---|
| ChEBI ID | 3165 |
|---|
| PubChem Compound ID | 439201 |
|---|
| Kegg Compound ID | C00306 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | M2MDB005413 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Postnov, V. N. Some aspects of the synthesis of organic functional groups on inorganic matrices. Napravl. Sintez Tverd. Veshchestv (1987), (2), 109-20. | | 2. Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. doi: 10.1038/nature07762. | | 3. Palkhiwala SA, Frishman WH, Warshafsky S: Bradykinin for the treatment of cardiovascular disease. Heart Dis. 2001 Sep-Oct;3(5):333-9. | | 4. https://www.ncbi.nlm.nih.gov/pubmed/?term=11799074 | | 5. https://www.ncbi.nlm.nih.gov/pubmed/?term=11975815 | | 6. https://www.ncbi.nlm.nih.gov/pubmed/?term=13446366 | | 7. https://www.ncbi.nlm.nih.gov/pubmed/?term=13811230 | | 8. https://www.ncbi.nlm.nih.gov/pubmed/?term=14597148 | | 9. https://www.ncbi.nlm.nih.gov/pubmed/?term=15105283 | | 10. https://www.ncbi.nlm.nih.gov/pubmed/?term=16680069 | | 11. https://www.ncbi.nlm.nih.gov/pubmed/?term=18127230 | | 12. https://www.ncbi.nlm.nih.gov/pubmed/?term=27318425 | | 13. https://www.ncbi.nlm.nih.gov/pubmed/?term=27328010 | | 14. https://www.ncbi.nlm.nih.gov/pubmed/?term=27432758 | | 15. https://www.ncbi.nlm.nih.gov/pubmed/?term=27445816 | | 16. https://www.ncbi.nlm.nih.gov/pubmed/?term=7859389 | | 17. https://www.ncbi.nlm.nih.gov/pubmed/?term=8723398 | | 18. https://www.ncbi.nlm.nih.gov/pubmed/?term=9403361 |
|
|---|